-
PDF
- Split View
-
Views
-
Cite
Cite
Arul E. Suthananthan, Sharnice A. Koek, Kishore Sieunarine, Cutaneous mucormycosis in an immunocompromised patient: a case report, Journal of Surgical Case Reports, Volume 2017, Issue 3, March 2017, rjx056, https://doi.org/10.1093/jscr/rjx056
Close - Share Icon Share
Abstract
Mucormycosis is a rare and highly aggressive fungal infection, with a potential to reach its fulminant phase rapidly. We report a case of a 73-year-old immunocompromised vasculopath with cutaneous mucormycosis. The disease resulted in eventual death despite aggressive surgical debridement, revascularization of his limb and amphotericin-B. This case highlights the need to recognize this disease early as a differential of a necrotic ulcer, to prevent a potentially avoidable fatality.
INTRODUCTION
Mucormycosis is a rare, highly aggressive fungal infection affecting the rhino-orbital, respiratory, gastrointestinal or cutaneous systems [1]. These highly aggressive infections caused by fungi of the mucorales order are often fatal. If recognized early, mucormycosis is a potentially treatable disease. We present a case of cutaneous mucormycosis caused by the species Rhizopus oryzae.
CASE REPORT
A male patient aged 73 years was admitted with two ulcers to his right lower leg and rest pain, which he attributed to a traumatic injury 8 weeks prior to presentation. He was known to have peripheral vascular occlusive disease with documented stenosis in both common iliac arteries (CIA), the left external iliac artery (EIA) and common femoral artery and occlusion of the right EIA.
His other relevant medical history includes a renal transplant, for which he takes regular prednisolone and azathioprine. He also had ischaemic heart disease with recent cardiac drug eluting stent insertion necessitating dual antiplatelet agent therapy and a coronary artery bypass graft in 1996.
Clinically he was aypyrexic with compromised circulation to his leg, which was pale but warm, and were absent of pulses on the affected side. There was a 1 cm wide ulcer located in the gaiter area, and a 3 cm wide ulcer located in the middle third of his lower leg posteriorly. Both lesions had a punched out appearance, with evident necrosis and no discharge or foul smell. He had a normal white cell count, a CRP count of 240 mg/L, potassium levels of 5.8 mmol/L and a creatinine level of 232 μmol/L.
His ankle brachial pressure index values were 0.23 on the right and 0.40 on the left. An arterial Doppler study showed no change in his occlusive disease, with patent, highly calcified vessels distally with poor flow. Doppler ultrasound studies of his transplanted kidney was normal.
To preserve renal function, a lower leg angiogram was performed using carbon dioxide. This showed an occluded right EIA and stenosed left CIA, with otherwise patent superficial femoral arteries bilaterally.
While undergoing medical assessment for a right EIA endarterectomy and left EIA stenting, the ulcers progressed in size necessitating a surgical debridement. Tissue samples from the initial debridement were sent for histopathology and microbiology.
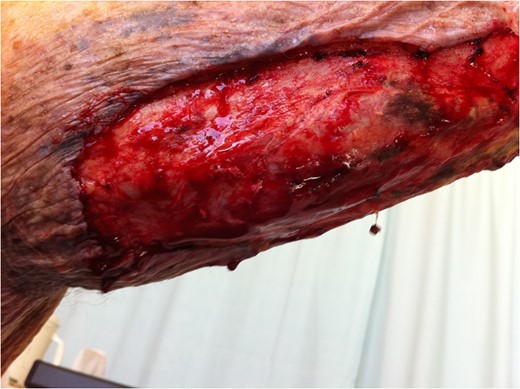
Extent of surgical debridement is illustrated, with new areas of necrosis evident within 24 h.
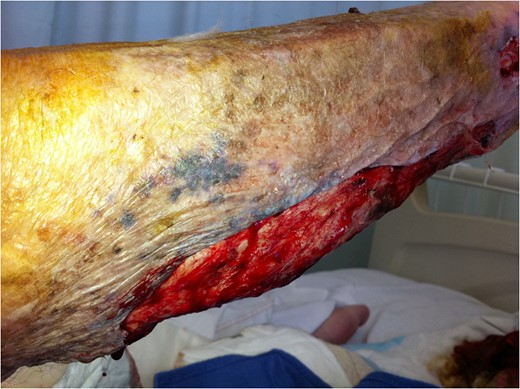
Extent of surgical debridement is illustrated, with new areas of necrosis evident within 24 h.
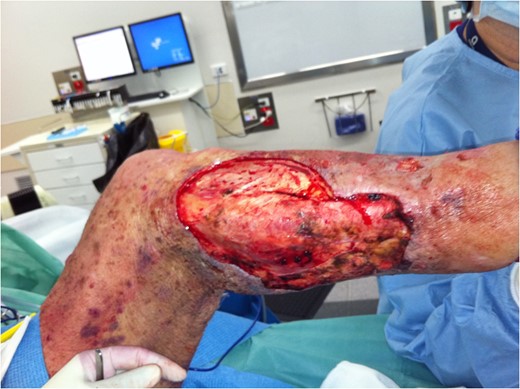
Further debridement underway. New areas of necrosis on medial upper thigh not illustrated.
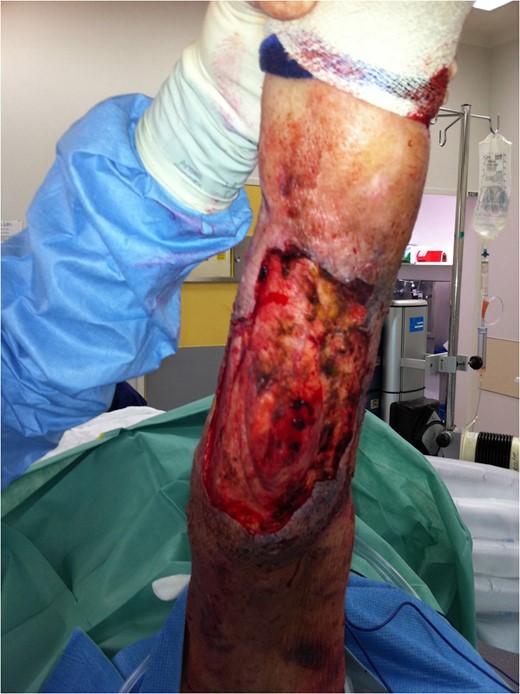
Further debridement underway. New areas of necrosis on medial upper thigh not illustrated.
Post operatively he was transferred to ICU, where over the course of 5 days, he had an improved blood supply to his right leg, with no further evidence of cutaneous necrosis. Nevertheless, he developed multi organ failure as a sequela of his disease and eventually died after 5 days.
DISCUSSION
Mucormycosis is a rare invasive fungal infection. The fungus of this patient was of the Mucorales order belonging to the class Zygomycete. Route of spread of Mucorales can occur via inhalation or ingestion of spores, or more importantly in immunocompromised hosts, via direct inoculation [2]. Cutaneous mucormycosis is the third most common manifestation of mucormycosis (19%) [2]. In a case series of 25 cases of mucormycosis, 10 cases consisted of cutaneous mucormycosis, out of which, 9 had local soft tissue damage [1].
Manifestation of cutaneous mucormycosis is variable and can present gradually or as a fulminant disease leading to dissemination [3]. Mortality of isolated cutaneous mucormycosis has been reported to be 31% but it can rapidly disseminate into deep organs leading to a mortality rate up to 94% [2, 4].
This case report illustrates the difficulty in detecting mucormycosis as well as the risk factors associated to the disease often making the disease more aggressive. Risk factors include immunosuppression, trauma, diabetes, intravenous drug abuse, prematurity, bone marrow transplantation and deferoxamine treatment [5, 6]. Once spores have invaded the host, the hosts defense systems are activated. In immunocompetent patients, phagocytosis of the spores prevents a fulminant course of the infection. Macrophages and neutrophils play an important role in this process. However, in immunocompromised individuals, lack of production and disruption of function of these immune cells lead to a rapid, progressive course of disease [1, 6]. Several of these risk factors including the use of long term corticosteroids, were present in this patient which raised the suspicion of mucormycosis. Biopsy samples should be attained early so treatment can be initiated. Pathological features include angioinvasion which initiates thrombosis and infarction of the affected surrounding tissues, leading to necrosis [5]. The presence of wide, twisted fungal hyphae within blood vessels, with necrosis of the tissues supplied by affected vessels are diagnostic for mucormycosis [7]. Microbiological studies can then delineate the species of fungi involved [3].
A multimodal approach in the management of cutaneous mucormycosis has been demonstrated to improve overall survival [3]. This involves reversing any risks and underlying contributing comorbidities, systemic antifungal treatment and aggressive surgical debridement [6]. Debridement optimizes cure rates by preventing further dissemination to deeper organs and manages the extensive necrosis occurring that may not be prevented by killing the organism with antifungals [8]. When combined with early, high dose systemic antifungal therapy, studies have shown that mortality can be reduced to <10% [3, 9]. Due to the resistance of mucorales, the antifungal agent of choice is typically amphotericin-B at high doses. However, due to its nephrotoxicity, renal function needs to be monitored [9, 10]. Novel regimens include the use of combination therapies (echinocandin or itraconazole) and adjunct treatments such as hyperbaric oxygen [3].
The key to early detection and management is having a high index of suspicion for the disease. Ulcers with necrotic edges are not uncommon, however, its rapidly progressive nature should warrant suspicion of this disease. Identification of known risk factors coupled with clinical findings and unresponsiveness to usual treatment, should prompt investigations. In this way, treatment can be initiated early to prevent a fulminant course of disease.
CONFLICT OF INTEREST STATEMENT
None declared.



