-
PDF
- Split View
-
Views
-
Cite
Cite
Pooja H. Patel, Joel Hayden, Randy Richardson, Ivemark syndrome: bronchial compression from anomalous pulmonary venous anatomy, Journal of Surgical Case Reports, Volume 2017, Issue 3, March 2017, rjx045, https://doi.org/10.1093/jscr/rjx045
Close - Share Icon Share
Abstract
Ivemark syndrome is a heterotaxy syndrome which affects multiple organs and affects roughly 1 in every 6000 deliveries. Specifically, it can cause total anomalous pulmonary venous return and cardiac defects, which ultimately lead to decreased life expectancy. In order to better understand the nature of cardiac structures, CT angiogram has been heavily relied upon as it also allows for 3D reconstruction and optimal visualization of those features. This specific case presents with an anomalous venous return accompanied by multi-organ right isomerism that was reconstructed with 3D CT angiogram to better visualize and understand the cardiopulmonary system, as well as contribute to a fund of knowledge in hopes of discovering a solution to this condition.
INTRODUCTION
Ivemark syndrome is a heterotaxy syndrome affecting multiple organs of the body, often presenting with congenital asplenia and cardiac problems [1, 2]. Commonly seen as right isomerism, typically there are two right-sided lungs and right-sided atria. The heart contains septal defects with right ventricular hyperplasia.
Additionally, the pulmonary venous return is irregular, long, and tortuous as it winds back to the heart. Life expectancy is heavily reduced with 10% of cases dying within the first 24 h and 80% dying within the first year of life [1]. These cardiac problems may include obstruction of pulmonary venous connections, seen in 48% of patients with heterotaxia and total anomalous pulmonary venous return (TAPVR) [3].
Here, we discuss an unusual case of TAPVR causing bronchial stenosis using cardiac CT angiogram to produce 3D functional figures displaying the anomalous pulmonary venous back to the left atrium.
CASE REPORT
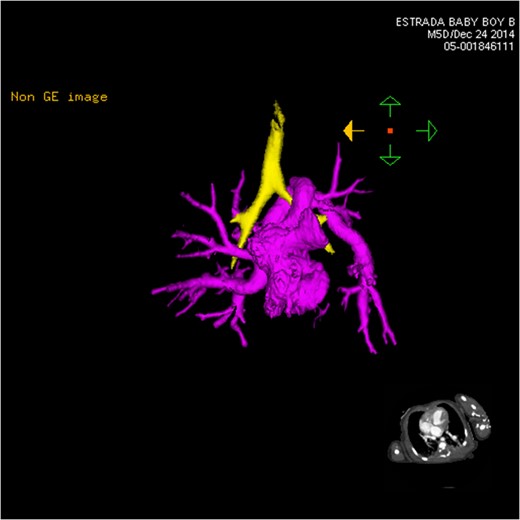
CT 3D showing anterior heart highlighting anomalous venous return (pink). The left venous vertical vein is shown passing over the left mainstem bronchus (yellow) as it enters the heart.
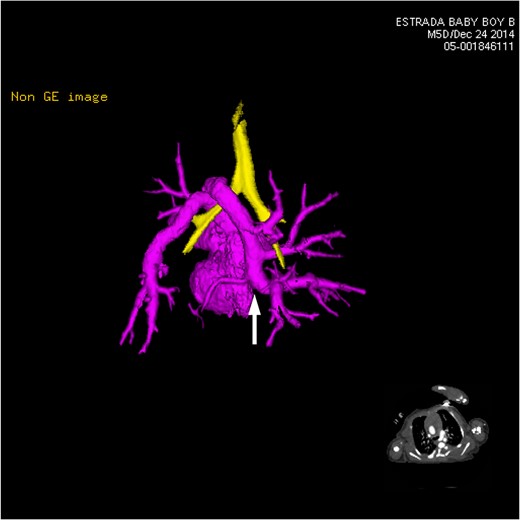
CT 3D showing posterior heart highlighting anomalous venous return (pink). Multiple pulmonary vein branches are shown conjoining together to form the left-sided vertical vein (white arrow). It dips under the carina and loops back over the left mainstem bronchus (yellow).
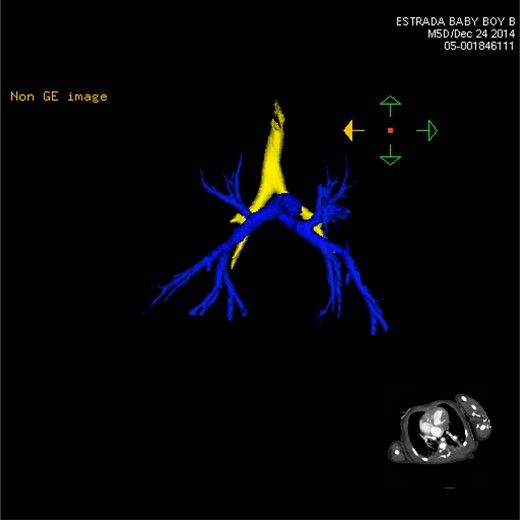
CT 3D showing anterior pulmonary arterial circulation (blue). Both left and right branches are nearly identical, characterized by the two right-sided lungs.
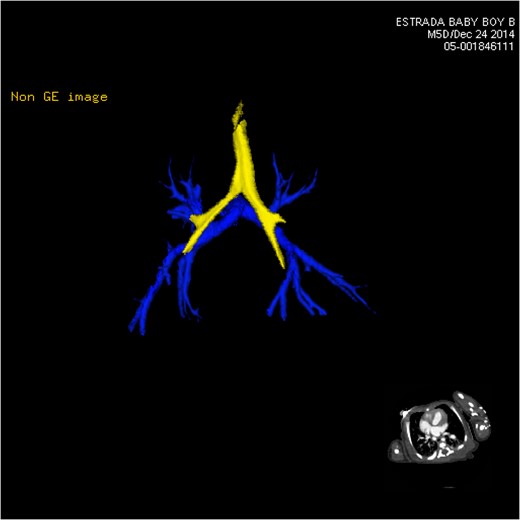
CT 3D showing posterior pulmonary arterial circulation (blue).
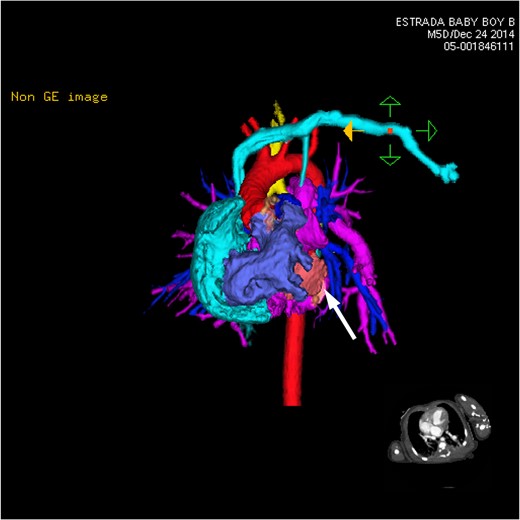
CT 3D showing anterior heart and circulation. The left-sided subclavian vein (light blue) brings blood to the right atrium (light blue). The right ventricle (dark purple) provides outflow to both the aorta (red) and the pulmonary arteries (dark blue). The multiple branches of the pulmonary veins (pink) can be seen coming together and wrapping over the left mainstem bronchus. The hypoplastic left ventricle (white arrow) can be seen small and shrunken slightly to the left of the large right atrium.
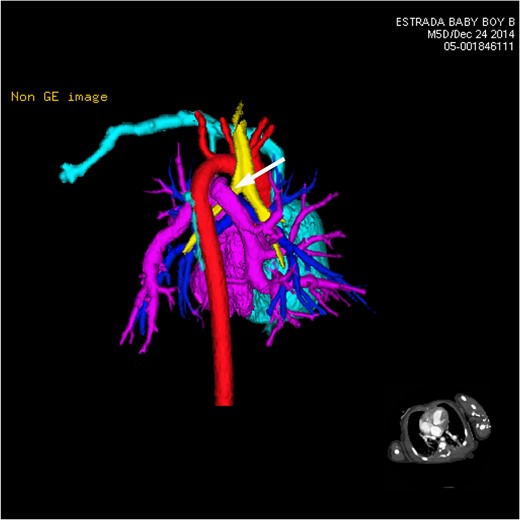
CT 3D showing posterior heart and circulation. This view focuses primarily on the anomalous branching and route of the pulmonary venous flow in relation to other vessels and airways nearby. The left-sided vertical vein can be seen traveling over the left bronchus (white arrow).
DISCUSSION
Ivemark syndrome is a unique form of heterotaxy, taking the right-sided form and thus resulting in no spleen development. Many times it is associated with anomalous pulmonary venous return, with the newly oxygenated blood traveling a long or ambiguous route back to the heart. Unfortunately, this kind of anomalous pulmonary venous return is different patient-to-patient contributing to the need for cardiac CT angiogram and subsequent 3D functional figures [3]. The concurrent septum primum ASD with corresponding VSD makes this issue very difficult to correct.
Due to the double-outlet right ventricle, there is a largely hypoplastic left ventricle and hyperplastic right ventricle. Both the pulmonary and systemic circulation falls upon the pumping action of the right ventricle, when typically the left ventricle handles the systemic circulation and the right ventricle handles the pulmonary circulation. Subsequently, the disuse of the left ventricle puts additional pressure on the right, increasing the pressure at which the right ventricle pumps out blood. Typically, the pulmonary circulation has lower blood pressure due to the nature of the small capillaries the blood passes through. The left ventricle usually pumps harder in order to provide circulation throughout the entire length of the body. With the right ventricle now serving as pumps for both types of circulation, there is the possibility of pulmonary edema and poor blood perfusion throughout the body [3]. Without efficient oxygenation of all of the blood and the adequate circulation of oxygenated blood out of the heart, the patient will likely suffer from chronic poor perfusion and shortness of breath, as well.
Additionally, the pulmonary veins curve over the left mainstem bronchus causing it to narrow compared to the right mainstem bronchus. With the heterotaxy seen in this patient, both lungs are right-sided causing the same slight angle of entry into the lungs by both mainstem bronchi. The slight narrowing of the left mainstem bronchus could lead to problems with breathing and physical activity.
As of yet, there have not been many attempts in permanently correcting these issues in Ivemark patients. Apart from a bidirectional Glenn operation to shunt blood from the vena cava to the pulmonary arteries and therefore bypass the right side of the heart, these patients must be kept under close observation for visible distress due to the heart condition.
CONFLICT OF INTEREST STATEMENT
None declared.



