-
PDF
- Split View
-
Views
-
Cite
Cite
Kenichi Amagasaki, Yutaka Takusagawa, Kyoko Kanehashi, Shoko Abe, Saiko Watanabe, Naoyuki Shono, Hiroshi Nakaguchi, Supratentorial acute subdural haematoma during microvascular decompression surgery: report of three cases, Journal of Surgical Case Reports, Volume 2017, Issue 2, February 2017, rjx004, https://doi.org/10.1093/jscr/rjx004
Close - Share Icon Share
Abstract
Supratentoiral haemorrhage during posterior fossa surgery is very rare. Authors report three cases of acute subdural haematoma occurred during microvascular decompression (MVD). Bleeding was observed in the suboccipital surgical area during operation but the origin of the bleeding was not confirmed intraoperatively in all cases. Decompression procedure was completed and immediate postoperative computed tomography revealed supratentorial subdural haematoma. This complication was observed during MVD in healthy young patients with hemifacial spasm in our cases. Flexion of the head with reduction of cerebrospinal fluid may have induced rotational movement of the cerebrum resulting in rupture of bridging veins, but no definitive mechanism that fulfils the clinical characteristics was clearly determined.
INTRODUCTION
Microvascular decompression (MVD) is widely accepted as an effective method to treat hemifacial spasm (HFS), trigeminal neuralgia (TN) and glossopharyngeal neuralgia (GPN), but the morbidity and mortality must be minimised because these conditions are not life-threatening. Unfortunately, serious complications can still occur in some patients, of which haemorrhagic event is one of the most dangerous complications and may result in significant morbidity and mortality. Intraoperative bleeding at a remote site is extremely rare, although some haemorrhagic complications following MVD have been reported [1–5]. We report three cases of intraoperative haemorrhage in the surgical field with unclear origin during MVD for HFS, in which computed tomography (CT) after the surgery confirmed supratentorial acute subdural haematoma (ASDH).
CASE DESCRIPTION
The three reported cases occurred among 1259 MVD procedures performed from 2006 to 2015, 852 for HFS, 386 for TN, 17 for GPN and 4 for tic convulsif, in 392 male and 867 female patients aged 19–86 years (mean age at operation 55.2 years). All operations for HFS were performed with a method described previously [6].
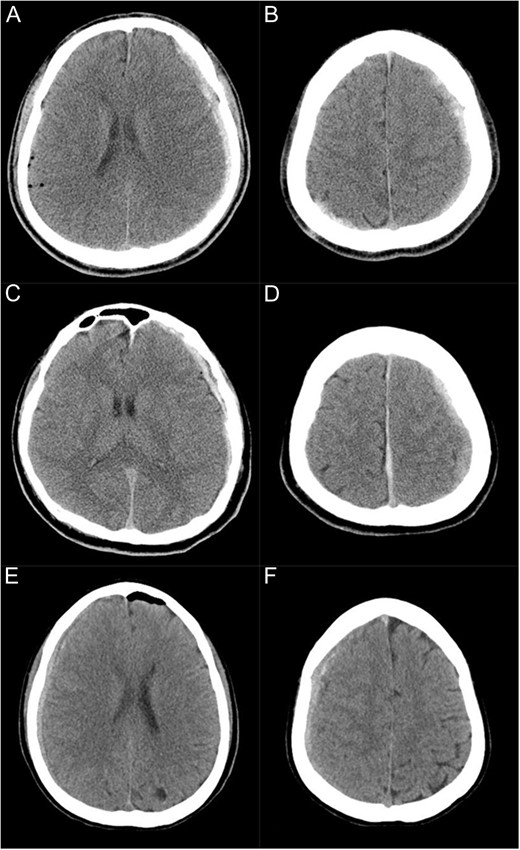
CT scans immediately after the surgery showing subdural haematoma in three cases (A and B: Case 1, C and D: Case 2, E and F: Case 3). (A and B) Subdural haematoma is present on the left supratentorial convexity as well as in the parieto-occipital area on the right side. (C and D) Subdural haematoma is present on the left supratentorial convexity and along the falx. (E and F) Subdural haematoma is present on the right supratentorial convexity.
| Case no. . | Age (yrs) . | Sex . | Side of HFS . | Dependent side at operation . | Site and side of ASDH . | Dominant side of venous drainage . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 37 | M | R | L | Bilateral (L > R) | R | Gerstmann's syndrome (incomplete), epilepsy |
| 2 | 33 | M | L | R | L and interhemi. | R | No deficit |
| 3 | 25 | F | L | R | R | L | No deficit |
| Case no. . | Age (yrs) . | Sex . | Side of HFS . | Dependent side at operation . | Site and side of ASDH . | Dominant side of venous drainage . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 37 | M | R | L | Bilateral (L > R) | R | Gerstmann's syndrome (incomplete), epilepsy |
| 2 | 33 | M | L | R | L and interhemi. | R | No deficit |
| 3 | 25 | F | L | R | R | L | No deficit |
Note: interhemi., interhemispheric fissure; L, left; R, right.
| Case no. . | Age (yrs) . | Sex . | Side of HFS . | Dependent side at operation . | Site and side of ASDH . | Dominant side of venous drainage . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 37 | M | R | L | Bilateral (L > R) | R | Gerstmann's syndrome (incomplete), epilepsy |
| 2 | 33 | M | L | R | L and interhemi. | R | No deficit |
| 3 | 25 | F | L | R | R | L | No deficit |
| Case no. . | Age (yrs) . | Sex . | Side of HFS . | Dependent side at operation . | Site and side of ASDH . | Dominant side of venous drainage . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 37 | M | R | L | Bilateral (L > R) | R | Gerstmann's syndrome (incomplete), epilepsy |
| 2 | 33 | M | L | R | L and interhemi. | R | No deficit |
| 3 | 25 | F | L | R | R | L | No deficit |
Note: interhemi., interhemispheric fissure; L, left; R, right.
DISCUSSION
Haemorrhagic events remoting from the craniotomy site can occur but the incidence across the tentorium cerebelli is rare. Remote cerebellar haemorrhage after supratentorial surgery is a rare but poorly understood complication [7]. The reverse phenomenon, such as supratentorial haemorrhage after infratentorial surgery, is even less common. A recent report documented four cases of supratentorial subdural haematoma following MVD [5]. In our cases, bleeding was observed during surgery, which is much more hazardous, and we could not find such a case in the literature. The report indicated that excessive CSF drainage and rotation and flexion of the neck in the lateral decubitus position should be considered as causative factors [5]. Slight vertex down is advantageous in MVD for HFS [4, 6], and the fact that this complication was observed in patients with HFS but not TN may support their discussion. On the other hand, CSF removal is usually dependent on the spontaneous excretion after the craniotomy; therefore, the rapidness of CSF removal rather than the amount may be related to this complication.
The haemorrhage must originate in either an artery or vein. A postmortem analysis of subdural haematoma revealed that the main bleeding points were either bridging veins draining into the superior sagittal sinus (SSS) or cortical arteries on the temporal lobes [8]. Surgical findings in Case 1 indicated no clear origin, suggesting that rupture of a cortical vein near the SSS was most likely because veins are more fragile than arteries and anatomically are exposed in the subdural space to the SSS [9]. Furthermore, a previous analysis of the mechanism of acute subdural haematoma suggested that rupture of bridging veins results from rotational acceleration [10].
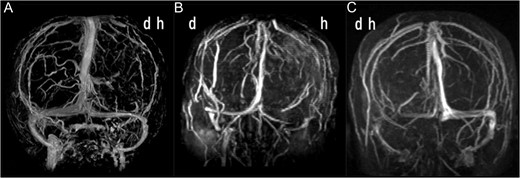
(A) Anteroposterior view of three-dimensional MR venogram after gadolinium enhancement of Case 1 showing the venous drainage is dominant on the right side. (B) MR venogram of Case 2 showing the venous drainage is dominant on the right side. (C) MR venogram of Case 3 showing the venous drainage is dominant on the left side. d, dependent side at operation; h, haematoma side.
Another important observation was that this complication occurred in younger patients in our series. The mean age of our patients with MVD was 55.2 years. The present three patients were younger. Previously, a 68-year-old patient presented with supratentorial acute subdural haematoma following MVD for HFS [1], and the recent reported cases were 49 years old and older. Despite the aetiology may be similar, the occurrence of this complication in only young patients remains unexplained. Accumulation of similar cases will be helpful for further understanding of the mechanism.
CONFLICT OF INTEREST STATEMENT
None declared.



