-
PDF
- Split View
-
Views
-
Cite
Cite
James A Milburn, John S Leeds, Steven A White, Endoscopic management of duodeno-ileal fistula secondary to diffuse B-cell lymphoma, Journal of Surgical Case Reports, Volume 2017, Issue 12, December 2017, rjx249, https://doi.org/10.1093/jscr/rjx249
Close - Share Icon Share
Abstract
Lymphoma arising in the gastrointestinal tract is relatively common and can affect multiple sites. The development of a gastrointestinal fistula secondary to lymphoma is very rare and has not previously been reported between the duodenum and ileum. This is the first reported care where a fistula secondary to lymphoma has been treated by an endoscopic covered duodenal stent occluding the defect rather than surgical intervention. This strategy permitted early commencement of curative intent chemotherapy which led to tumour shrinkage and fistula closure.
INTRODUCTION
Primary gastrointestinal lymphoma (PGIL) accounts for 30–40% of extra-nodal malignant lymphoma cases and up to 10–15% of all non-Hodgkin’s lymphoma (NHL) [1]. Temporal data series suggest the incidence of PGIL is increasing in the Western world although the reasons are uncertain. Internal entero-entero fistulation between intestinal segments can be due to multiple aetiologies although there are sparse reports of lymphoma as a primary cause. Experience with the endoscopic management of upper gastrointestinal (GI) fistulas is limited as an operative intervention is often performed. We report the case of an 80-year-old man who presented with symptomssds attributable to a duodeno-ileal fistula secondary to diffuse B-cell lymphoma leading to the first reported case of endoscopic management.
CASE REPORT
An 80-year-old male presented to the emergency department with worsening diarrhoea and faeculent vomiting on a background of significant weight loss and dyspepsia. He passed 8–10 watery stools per day which were noted to contain recently ingested foodstuffs. Past medical history included hypertension, diabetes, bronchiectasis and obesity. Four years previously he had undergone laparotomy for common bile duct exploration and cholecystectomy through a supraduodenal approach. Prior endoscopic retrograde cholangiopancreatography was unsuccessful due to multiple large calculi although no duodenal abnormalities were noted either before or after this intervention.
Initial upper GI endoscopy failed to enter the duodenum due to residue within the stomach with the appearance and odour of faeces noted. Abdominal computed tomography (CT) suggested a cavity with adherent ileum adjacent to the second part of the duodenum (Fig. 1). A contrast study was then undertaken demonstrating rapid flow of contrast into the terminal ileum and caecum originating from the duodenum (Fig. 2). Repeat upper GI endoscopy demonstrated an abnormal fungating fistulous communication between the duodenum and terminal ileum which permitted the full insertion of the endoscope (Figs 3 and 4). Biopsies were consistent with diffuse B-cell lymphoma (DLBCL) in accordance with the WHO classification.
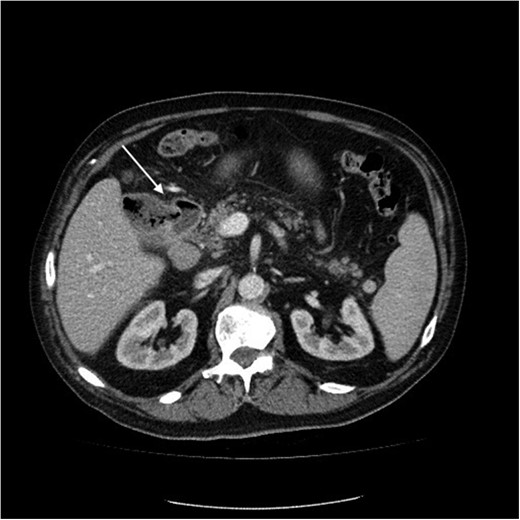
Computed tomography image of duodenal-ileal mass (arrow indicates cavity).

Oral contrast study showing transit of barium into the ileum from a duodenal source through a cavity (arrow indicates cavity arising from duodenum).
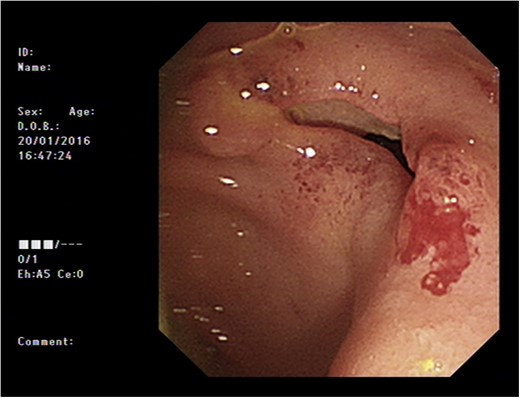
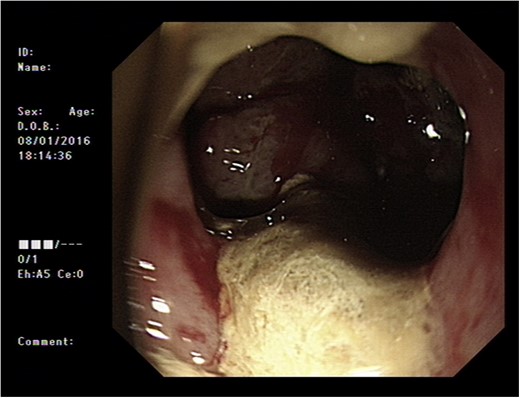
The patient was commenced on a combination of parenteral nutrition with passage of a nasojejunal tube beyond the fistula with cautious introduction of enteral feeding. He was assessed for surgical intervention but due to his comorbidities this was considered very high risk. He therefore underwent insertion of a fully covered self expanding metal stent (60 × 20 mm2 duodenal stent, Tawoong Medical, South Korea) to cover the defect.
He symptomatically improved and commenced combination chemotherapy (R-GCVP Rituximab, Gemciabine, Cyclophosphamide, Vincristine and Prednisolone). Subsequent radiological and upper GI endoscopy showed sealing of the fistula at 3 months. (Fig. 5) The patient was able to resume a full oral diet when the stent was removed. Six months following diagnosis the patient continues to do well with a good response to chemotherapy with planned CT surveillance.
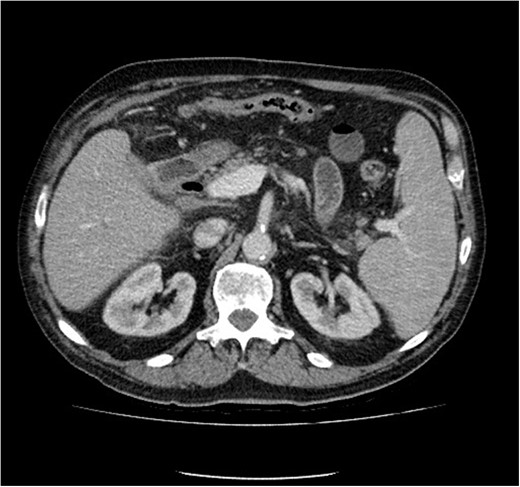
Computed tomography image showing improvement in appearances (stent removed).
DISCUSSION
PGLI lymphoma can affect any segment of the GI tract with a variety of pathological subtypes exhibiting a global variation. The most frequent PGLI arise from proliferating peripheral B-lymphocytes and over half lead to DLBCL [2]. In a Western population approximately half of PGLI arise in the stomach, with a quarter in the small intestine and 10% the colon, with rare presentations in the oesophagus, liver or pancreas. Up to 15% of patients will have multiple sites of involvement in the GI tract [3].
Haldane reported the first case of a malignant GI fistula due to adenocarcinoma in 1862 while lymphoma was first implicated as aetiology in 1946 [4, 5]. A 2005 review of the literature concluded that <20 entero-entero fistulae secondary to lymphoma had been reported primarily treated surgically [6]. These intra-abdominal internal lymphomatous fistulae are more commonly jejuno-colic or gastro-colic [6, 7].
No previous cases of duodeno-ileal fistulae secondary to PGLI have been reported in the literature suggesting this is the first reported case. Colo-enteric fistulae between duodenum and colon often arise due to their anatomic proximity although it is unclear why the ileum in this patient was in proximity to the second part of the duodenum. This would normally suggest fistulation through the mesocolon although given the previous upper abdominal surgery the ileum could have migrated close to the duodenum post operatively.
It is difficult to ascertain whether the DLBCL originated in the duodenum or terminal ileum. The ileum is the commonest site of origin for small bowel GI lymphoma therefore this case is likely to represent a DLBCL originating in the ileum fistulating into the duodenum [8]. Follicular and mantle cell lymphoma of the duodenum but without fistulation have been reported although would be different histological subtype to this case [9].
Macroscopic subtypes of PGIL have been described within the small bowel and in our case was likely an ulcerative variant as these are commonest among DLBCL and would predispose to fistulation.
The management of PGIL has evolved with chemotherapy, radiotherapy and immunotherapy combinations the optimal treatment with surgery often restricted to the treatment of complications including fistulae.
Upper GI surgery for PGIL can result in significant peri-operative complications leading to a high risk of morbidity and potential mortality [1]. Duodenal fistulas are particularly difficult to manage because of the risk of high output fluid loss and sepsis while causing distressing symptoms including faeculent vomiting. However, surgery remains advocated by some groups especially for intestinal (non-gastric) tumours reporting improved outcomes with adjuvant chemotherapy rather than chemotherapy alone [3]. Furthermore, indolent tumour including Mantle or Follicular subtypes where chemotherapy is less effective may also benefit from resection [1].
In this case, surgery would have required a pancreaticoduodenectomy with a right hemicolectomy conferring a high risk of mortality and morbidity in this elderly patient. Surgery was avoided by the use of a covered duodenal stent which simultaneously excluded the defect allowing restoration of enteral nutrition and allowed early introduction of chemotherapy agents avoiding delay to treatment in postoperative recovery. The use of duodenal stents to treat fistulating conditions of the GI tract including the duodenum has previously been reported although often this is in palliative setting [10].
Our report has described the novel use of a duodenal stent as bridging therapy to facilitate early introduction of chemotherapy in a complex patient. Advanced endoscopic techniques can be a useful therapeutic modality in PGIL cases to avoid surgery and improve outcomes.
CONFLICT OF INTEREST STATEMENT
None declared.



