-
PDF
- Split View
-
Views
-
Cite
Cite
Khaled Abdelwahab, Omar Hamdy, Mona Zaky, Nirmeen Megahed, Saleh Elbalka, Mohamed Elmetwally, Adel Denewer, Breast fibromatosis, an unusual breast disease, Journal of Surgical Case Reports, Volume 2017, Issue 12, December 2017, rjx248, https://doi.org/10.1093/jscr/rjx248
Close - Share Icon Share
Abstract
Fibromatosis is a benign tumor that rarely affects the breast and is an unusual site for its occurrence. Whilst the definite etiology of breast fibromatosis is unclear, it may present itself following surgical trauma or silicone implant. Wide local excision with adequate safety margins is considered the standard of care. We review three cases of breast fibromatosis who were presented to and operated in the Oncology center, Mansoura universty (between April 2014 and August 2016). Two of these cases underwent wide local excision and primary closure of the defect whilst the other one was reshaped by mini latismuss dorsi flap.
INTRODUCTION
Fibromatosis is widely known as a benign tumor that poses high recurrence rate even with adequate surgical resection. The breast is an unusual site for its occurrence with reported incidence of ~0.2% of breast tumors [1, 2]. It can arise from fibroblasts and myofibroblasts in the breast parenchyma or from the pectoralis musculoaponeurotic layer [3, 4]. The definite etiology of it is unclear, however, it was reported as a consequent of surgical trauma or silicone implant as well as being an association with Gardener’s syndrome [5, 6]. We report three cases of breast fibromatosis in our surgical unit, Mansoura oncology center.
CASE 1
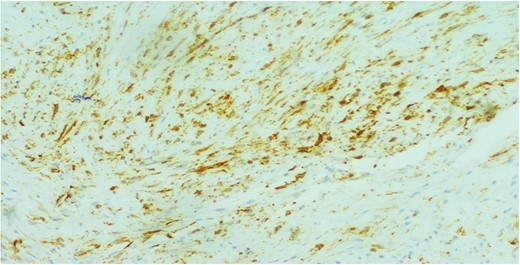
A 26-year-old female presented to our unit with recurrent right breast lump that was excised 6 years before with no available documented data. Physical examination revealed hard painless swelling occupying the medial quadrant of the right breast below the site of the old incision with no clinically palpable axillary LNs. Ultrasonography reported an ill-defined heterogeneous hypoechoic soft tissue lesion. Core needle biopsy revealed dense collagen bundles. Excision of the mass was done and paraffin sections stained by Hematoxylin and Eosin (H&E) showed benign tumoral proliferation formed of spindle cells arranged in small clustered fasicles separated by thick collagen bands (Fig. 1). Further IHE studies confirmed the diagnosis of fibromatosis supported by positive nuclear staining for B-catenin (Fig. 2). And 18 months later, she came with abdominal wall swelling, underwent CT scanning and found to be multiple soft tissue masses at the lower anterior abdominal muscles at the right lumbar and iliac regions.The largest measured ~13 × 10 cm. Core needle biopsy revealed spindle cell proliferation consistent with fibromatosis confirmed by positive reaction for SMA (Fig. 3) and B-catenin. Lesions were excised with free margins and reconstruction of the abdominal wall was carried out using a double face mesh. Final pathology report was consistent with fibromatosis confirmed by positive reaction for SMA and B-catenin.The patient had smooth postoperative course.
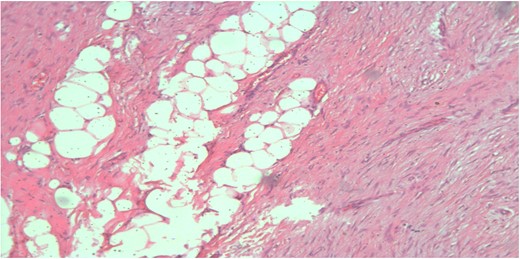
Spindle cell proliferation formed of bland looking cell arranged in short fascicles with dissection of breast fat lobules at the periphery (H&E, ×100x).
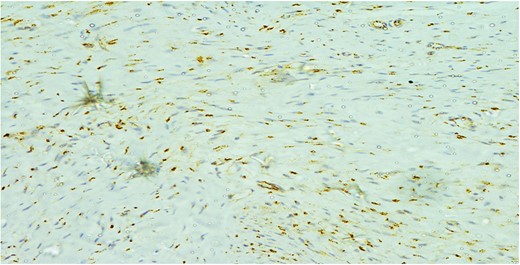
The cells showed diffuse positive nuclear staining for B-catenin (×200).
CASE 2
A 28-year-old female presented to us with a slowly progressive painless right breast lump. Physical examination showed a firm painless right breast swelling with ill-defined borders occupying the upper inner quadrant of the right breast with no clinically palpable axillary LNs. The lesion was described by ultrasonography as a large irregular soft tissue mass extending from 11 to 3 o'clock of right breast at areolar edge inducing slight parenchymal distortion measuring ~5 × 3 cm (BIRADS 4b). Trucut needle biopsy from the mass revealed bland looking spindle cell proliferation with few breast ducts in between and was diagnosed as benign spindle cell proliferation consistent with benign phyllodes. Wide local excision of the mass was completed and was sent for frozen section revealing benign spindle cell lesion. The defect was reconstructed by mini latissimus dorsi flap. Pathology report of the paraffin sections stained by H&E revealed spindle cell proliferation mostly fibromatosis. Further immunohistochemical studies led to the diagnosis of fibromatosis confirmed by positive nuclear staining for B-catenin, focal positive for SMA, negative CK and CD34. The patient is followed now for 2 years with no apparent recurrence.
CASE 3
A 33-year-old female, complaining also from slowly progressive painless left breast lump. Physical examination revealed painless swelling in the axillary tail of the left breast measuring clinically ~3 × 1 cm with no clinically palpable axillary LNs. The lesion was described by ultrasonography as a well-defined irregular bordered soft tissue mass at 2 o'clock zone C measuring ~4 × 1.4 cm2 (BIRADS 4b). Trucut biopsy revealed spindle cell proliferation. Again, wide local excision of the mass and primary closure of the defect was carried out. Pathology report of the paraffin sections stained by H&E revealed tumoral proliferation formed of spindle cells running in storform and herring bone pattern. The cells exhibited mild atypia and pleomorphism. The slides prepared from all surgical margins were free from tumor tissue. Further immunohistochemical studies led to the diagnosis of fibromatosis confirmed by focal positivity for B-catenin and negativity for SMA, S100. The patient had smooth postoperative course and free short term follow up.
DISCUSSION
Fibromatosis is a rare tumor with locally aggressive behavior and high incidence of local recurrence [7]. Various causes have been accused of being its etiology including genetic and endocrinal factors as well as surgical trauma [8, 9]. For treatment of fibromatosis, wide local excision with adequate safety margins is considered the standard of care.
The breast is an unusual site for occurrence of desmoid type fibromatosis with few cases reported in literature. It represents 0.2% of all breast tumors and 4% of all extra-abdominal desmoid tumors. Bilaterality has been reported in 4% of cases [1, 3, 4]. Most of these cases were reported in young fertile females with rare cases reported in males. The reported risk factors include surgical trauma, silicone implants as well as in association with Gardener’s syndrome [5]
Clinically, it is presented as a firm mass with skin dimpling and nipple retraction in superficial and retroareolar tumors. Neither nipple discharge nor axillary lymphadenopathy commonly occur with it [5, 10]. Our cases showed neither nipple retraction nor skin affection.
Mammography presents irregular walled and highly dense lesion with no calcifications mimicking sometimes breast carcinoma [11]. Radiologic evaluation of our cases revealed large irregular soft tissue masses inducing slight parenchymal distortion. The second and the third cases were classified as BIRADS 4b.
Microscopic examination of desmoid tumor usually reveals the characteristic irregular bundles of spindle cells with regular nuclei surrounded by abundant collagen Giant cells, macrophages and lymphocytes are noticed mostly peripherally [2]. Pathological evaluation of our cases revealed tumoral proliferation formed of spindle cells running in storiform and herring bone pattern. Immunohistochemical staining usually shows positivity for B-catenin in 70–80% of cases despite being nonspecific for fibromatosis [10]. All our cases showed positivity for B-catenin IHC.
CONCLUSION
Though rare, we should consider the diagnosis of breast fibromatosis when we have a past history of previous surgeries or use of silicone implants. Such consideration will be confirmed by the appropriate IHC study.
CONFLICT OF INTEREST STATEMENT
None declared.



