-
PDF
- Split View
-
Views
-
Cite
Cite
Keita Kouzu, Hironori Tsujimoto, Shuichi Hiraki, Risa Takahata, Yoshihisa Yaguchi, Isao Kumano, Hiroyuki Horiguchi, Shinsuke Nomura, Ken Nagata, Manabu Harada, Hiromi Nagata, Takao Sugihara, Yusuke Ishibashi, Yujiro Itazaki, Satoshi Tsuchiya, Suefumi Aosasa, Kazuo Hase, Junji Yamamoto, Hideki Ueno, A case of pneumatosis intestinalis during neoadjuvant chemotherapy with cisplatin and 5-fluorouracil for esophageal cancer, Journal of Surgical Case Reports, Volume 2017, Issue 11, November 2017, rjx227, https://doi.org/10.1093/jscr/rjx227
Close - Share Icon Share
Abstract
Pneumatosis intestinalis (PI) is a relatively rare disease. A 70-year-old man with stage II squamous cell carcinoma of the middle thoracic esophagus was administered cisplatin plus 5-fluorouracil (CF) therapy as neoadjuvant chemotherapy. On Day 14 of the first course of CF therapy, he complained of acute abdominal pain. Computed tomography (CT) revealed PI of the entire colon and a small air bubble in the mesentery. A colonoscopy revealed that there was no finding suggestive of ischemia. Because there was no sign of peritoneal irritation, conservative treatment was selected. On Day 7 after PI diagnosis, CT indicated the disappearance of PI. The patient underwent a radical esophagectomy. Intraoperative laparoscopic findings showed the serosa of the colon to be intact. The patient was discharged without any complications. It is important to take into account that CF therapy may cause PI and that PI can be treated conservatively.
INTRODUCTION
Pneumatosis intestinalis (PI) is characterized by the presence of gas within the gastrointestinal tract wall. Although PI often represents a benign condition, immediate surgery may be required in some life-threatening situations, such as bowel obstruction, perforation or ischemia. Classically, PI can be subdivided into two distinct groups: a primary PI that represents 15% of cases and a secondary PI that represents 85% of cases [1]. A secondary PI has been attributed to immunological disturbances, bowel mucosal disruptions, and intra-abdominal pathologies and frequently requires an urgent laparotomy. In contrast, a primary PI is characterized by intramural gas that is cystic and benign in nature and does not always require an urgent laparotomy. Although PI may occur in association with acquired immunodeficiency, transplant status, inflammatory bowel disease, intestinal ischemia, colitis, trauma or cancer treatment [2], the exact etiology of primary and secondary PI remains unclear.
Here, we describe a case of PI during neoadjuvant chemotherapy (NAC) with cisplatin and 5-fluorouracil (CF) for advanced esophageal cancer that was successfully treated conservatively.
CASE REPORT
A 70-year-old man visited to our hospital with persistent difficulty in swallowing. He was diagnosed with stage II esophageal cancer by endoscopy, computed tomography (CT) and fluorodeoxyglucose-enhanced positron emission tomography. Because his oral intake was insufficient due to the obstruction of the tumor, he underwent a laparoscopic jejunostomy prior to NAC [3]. At Day 10 after the initiation of the first course of NAC with CF, he experienced slight nausea and abdominal distention, which were treated with antiemetics. At Day 14 after the initiation of NAC, he had severe abdominal pain with lower abdominal tenderness. His body temperature was 37.7 °C and heart rate was 119 beats/min. Blood tests revealed an elevation in the C-reactive protein level (3.7 mg/dL) and renal dysfunction. CT scan revealed the presence of gas within the wall of the entire colon and a small amount of free air in the mesentery (Fig. 1A). Thus, he was diagnosed with PI. A colonoscopy revealed an edema of the mucosa with a white coat, which was not observed before NAC (Fig. 2). Because there were no signs of intestinal ischemia and peritoneal irritation, we treated him conservatively; treatments included cessation of enteral nutrition, administration of meropenem hydrate and selective decontamination of the digestive tract, and oxygenation. The day after the initiation of these therapies, his abdominal pain improved. CT at Day 7 after the PI diagnosis showed that the gas within the wall had disappeared (Fig. 1B). Because NAC with CF was highly suspected to have caused PI, we abandoned the second course of CF therapy. The therapeutic effect of NAC resulted in a stable disease. We carried out video-assisted thoracoscopic esophagectomy on Day 14 after the initiation of NAC. An intraoperative laparoscopy showed no significant findings in the serosa of the colon and no intra-abdominal abnormal adhesions. He was discharged from our hospital without any complications, including PI recurrence.
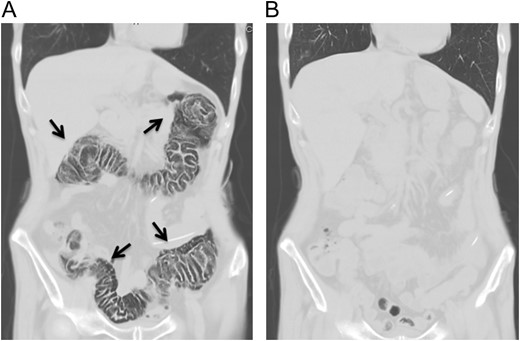
Abdominal computed tomography at the onset of abdominal pain revealed pneumatosis intestinalis of the colon. No free air was detected (A). Computed tomography 7 days after the diagnosis of pneumatosis intestinalis did not show any gas within the wall of colon (B).
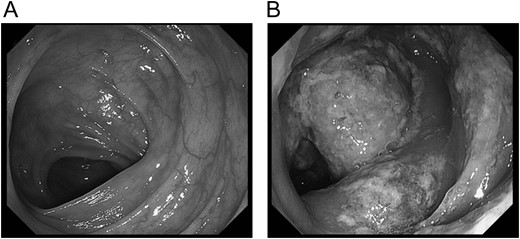
Colonoscopy at the onset of abdominal pain. In colonoscopy on the day of onset of pneumatosis intestinalis, the edema of the mucous membrane and white coat were observed.
DISCUSSION
PI is defined by the presence of gas within the intestinal wall. Most PI symptoms are non-specific, and thus image findings are necessary to diagnose PI; an abdominal X-ray, ultrasonography and CT are useful diagnostic tools.
The pathogenesis of PI is still a debatable topic. Some proposed theories have thought it to be multifactorial. Two major explanations as to how the gas gets into the intestinal wall have been supported by mechanical and bacterial theories [4]. The mechanical theory hypothesized that the increased intraluminal pressure of the intestine allows gas to penetrate into the submucosal space through any mucosal disruption. The bacterial theory hypothesized that gas-forming bacilli enter the submucosa through mucosal breaks or increased mucosal permeability and subsequently form gas within the bowel wall.
It is well known that molecular targeted drugs frequently cause PI. Shinagare et al. [5] reported that 10 out of 48 patients with molecular targeted therapy such as Bevacizumab and sunitinib had PI; two patients had abdominal pain and eight patients had no clinical symptoms. In contrast to molecular targeted drugs, there are few reports of PI being associated with chemotherapy for malignancies [6], especially those caused by CF therapy. Galm et al. [7] reported that 5-fluorouracil induced alternation in the local mucosal blood flow and had thrombogenic and vasospasmtic effects on the epithetium that resulted in PI. Cisplatin has been reported to enhance gastrointestinal toxicity of irinotecan, but there is no report that cisplatin induces gastrointestinal toxicity alone [8]. Thus, a potential mechanism of PI induced by CF therapy may be a mucosal disorder caused by 5-fluorouracil, which was exacerbated by cisplatin.
To our knowledge, six reported cases, including the present case, had PI associated with chemotherapy without molecular targeted drugs (Table 1). Of these, two patients had PI in their small intestine and four had it in their colon. No patients had PI in the entire colon, except for our case. The interval between the appearance of PI and the start of chemotherapy ranged from 6 days to 2 months, and five out of six patients were treated conservatively. Kung et al. [9] performed an emergent laparotomy because of a high index of suspicion for ischemia and bowel necrosis that had portal venous gas (PVG) as well as PI. PVG was associated with intestinal ischemia and necrosis in 75% of reported cases. Because our case had no signs of intestinal ischemia or peritoneal irritation such as lactic academia, we performed conservative therapy. If there is a suspicion of PI associated with intestinal ischemia, a laparoscopic exploration should be considered to rule out bowel perforation and ischemia [10].
Reported cases of pneumatosis intestinalis during chemotherapy without molecular target drugs.
| Authors . | Ref. number . | Age . | Sex . | Site of PI . | Diagnosis . | Chemotherapy agents . | Period after finishing chemotherapy . | Treatment of PI . |
|---|---|---|---|---|---|---|---|---|
| Vijayakanthan | #9 | 48 | M | Small intestine | Non-Hodgkin’s lymphoma |
| 6 days | Conservative (antibiotics) |
| Gray | #13 | 69 | M | S/C | Lung cancer |
| 8 days | Conservative (oxygen) |
| Shin | #17 | 18 | M | A/C, T/C | Leukemia |
| 2 months | Conservative (oxygen, antibiotics) |
| Mimatsu | #18 | 76 | M | Small intestine | Rectal cancer |
| NR | Conservative (oxygen, antibiotics) |
| Kung | #19 | 69 | M | A/C | Esohageal cancer |
| NR | Surgical intervention |
| Our case | – | 71 | M | Entire colon | Esohageal cancer |
| 14 days | Conservative (oxygen, antibiotics) |
| Authors . | Ref. number . | Age . | Sex . | Site of PI . | Diagnosis . | Chemotherapy agents . | Period after finishing chemotherapy . | Treatment of PI . |
|---|---|---|---|---|---|---|---|---|
| Vijayakanthan | #9 | 48 | M | Small intestine | Non-Hodgkin’s lymphoma |
| 6 days | Conservative (antibiotics) |
| Gray | #13 | 69 | M | S/C | Lung cancer |
| 8 days | Conservative (oxygen) |
| Shin | #17 | 18 | M | A/C, T/C | Leukemia |
| 2 months | Conservative (oxygen, antibiotics) |
| Mimatsu | #18 | 76 | M | Small intestine | Rectal cancer |
| NR | Conservative (oxygen, antibiotics) |
| Kung | #19 | 69 | M | A/C | Esohageal cancer |
| NR | Surgical intervention |
| Our case | – | 71 | M | Entire colon | Esohageal cancer |
| 14 days | Conservative (oxygen, antibiotics) |
S/C, Sigmoid colon; A/C, ascending colon; T/C, transverse colon; NR, not referred.
Reported cases of pneumatosis intestinalis during chemotherapy without molecular target drugs.
| Authors . | Ref. number . | Age . | Sex . | Site of PI . | Diagnosis . | Chemotherapy agents . | Period after finishing chemotherapy . | Treatment of PI . |
|---|---|---|---|---|---|---|---|---|
| Vijayakanthan | #9 | 48 | M | Small intestine | Non-Hodgkin’s lymphoma |
| 6 days | Conservative (antibiotics) |
| Gray | #13 | 69 | M | S/C | Lung cancer |
| 8 days | Conservative (oxygen) |
| Shin | #17 | 18 | M | A/C, T/C | Leukemia |
| 2 months | Conservative (oxygen, antibiotics) |
| Mimatsu | #18 | 76 | M | Small intestine | Rectal cancer |
| NR | Conservative (oxygen, antibiotics) |
| Kung | #19 | 69 | M | A/C | Esohageal cancer |
| NR | Surgical intervention |
| Our case | – | 71 | M | Entire colon | Esohageal cancer |
| 14 days | Conservative (oxygen, antibiotics) |
| Authors . | Ref. number . | Age . | Sex . | Site of PI . | Diagnosis . | Chemotherapy agents . | Period after finishing chemotherapy . | Treatment of PI . |
|---|---|---|---|---|---|---|---|---|
| Vijayakanthan | #9 | 48 | M | Small intestine | Non-Hodgkin’s lymphoma |
| 6 days | Conservative (antibiotics) |
| Gray | #13 | 69 | M | S/C | Lung cancer |
| 8 days | Conservative (oxygen) |
| Shin | #17 | 18 | M | A/C, T/C | Leukemia |
| 2 months | Conservative (oxygen, antibiotics) |
| Mimatsu | #18 | 76 | M | Small intestine | Rectal cancer |
| NR | Conservative (oxygen, antibiotics) |
| Kung | #19 | 69 | M | A/C | Esohageal cancer |
| NR | Surgical intervention |
| Our case | – | 71 | M | Entire colon | Esohageal cancer |
| 14 days | Conservative (oxygen, antibiotics) |
S/C, Sigmoid colon; A/C, ascending colon; T/C, transverse colon; NR, not referred.
Although there are several reports that PI was induced by jejunal feeding or colonoscopy, these causes were not suitable in this case because the site of PI was the colon but not the small intestine and colonoscopy was not performed before the occurrence of PI.
In conclusion, it is important to consider that chemotherapy without molecular targeted agents, such as CF regimen, may cause PI in the large bowel, and PI occurred during chemotherapy lacking abdominal symptoms that lead to suspecting intestinal ischemia or perforation can be treated conservatively.
CONFLICT OF INTEREST STATEMENT
The author has no financial conflicts of interest to disclose concerning the report.
Funding
This study was supported JSPS KAKENHI Grant no. 15K10042.
REFERENCES
Author notes
All authors meet the ICMJE authorship criteria.
- ischemia
- fluorouracil
- acute abdomen
- computed tomography
- acute abdominal pain
- squamous cell carcinoma
- colonoscopy
- cisplatin
- cystic fibrosis
- esophageal cancer
- esophagectomy
- intraoperative care
- laparoscopy
- mesentery
- pneumatosis cystoides intestinalis
- serous membrane
- colon
- diagnosis
- peritoneum
- chemotherapy, neoadjuvant
- thoracic esophagus
- conservative treatment
- rare diseases



