-
PDF
- Split View
-
Views
-
Cite
Cite
Patrick Thornley, Stephan Oreskovich, John Provias, Jamie Silva, Kesava Reddy, Aleksa Cenic, Multiple lobar intracerebral hemorrhage and cerebral amyloid angiopathy in the setting of ApoE Є2: a case report of the disease course and call for improved treatment modalities, Journal of Surgical Case Reports, Volume 2017, Issue 10, October 2017, rjx208, https://doi.org/10.1093/jscr/rjx208
Close - Share Icon Share
Abstract
We describe a case of multiple cerebral amyloid angiopathy-associated intracerebral hemorrhages (ICH) occurring at multi-focal lobar sites of the brain. A review and discussion of the current literature on the importance of Apolipoprotein E (ApoE) genotyping in prediction of ICH outcome and recurrence follows.
INTRODUCTION
Cerebral amyloid angiopathy (CAA), a vasculopathic change caused by deposition of beta-amyloid protein fibrils typically confined to cerebral cortex and leptomeningeal vessels, is implicated in 12–15% of intracerebral hemorrhages (ICH) among the elderly [1]. It is commonly accepted that beta-amyloid deposition leads to necrosis and subsequent vessel weakening, increasing the possibility of ICH [1]. Additionally, CAA appears to have a strong genetic link via the Apolipoprotein E (ApoE) gene (Є2 and Є4) [1, 2].
The following case describes a patient with multiple ICH at different lobar sites, with a pathological diagnosis of CAA confirmed by operative pathology and a presumable CAA cause for other ICH not biopsied. We also review and discuss current medical literature on CAA-associated ICH with emphasizing the importance of ApoE genotyping in prediction of ICH outcome and recurrence.
CASE REPORT
A 66-year-old Italian female with a medical history of hypertension, asthma and hysterectomy presented with sudden severe right-sided headache, blurred vision and vomiting. On presentation, the patient indicated compliance with current medical therapy, including Cardizem-SR, Ipratropium Bromide/Salbutamol, Fluticasone Propionate and Estradiol Gel. The patient disclosed that her mother suffered ‘multiple strokes’; however, further family history was unavailable.
Clinical progress
First ICH: Blood pressure (BP) was 145/75 on admission. Neurological exam revealed left homonymous hemianopsia. She denied any history of trauma, and ensured compliance with her anti-hypertensive medications. Computed tomography (CT) scan of her head revealed an ICH in the right parieto-occipital region (Fig. 1A). Routine coagulation parameters (INR, PTT and platelets) were unremarkable. Cerebral angiogram was performed and revealed no evidence of a vascular abnormality. The patient was discharged home with a resolving left homonymous hemianopsia. A follow-up CT revealed an evolving ICH.
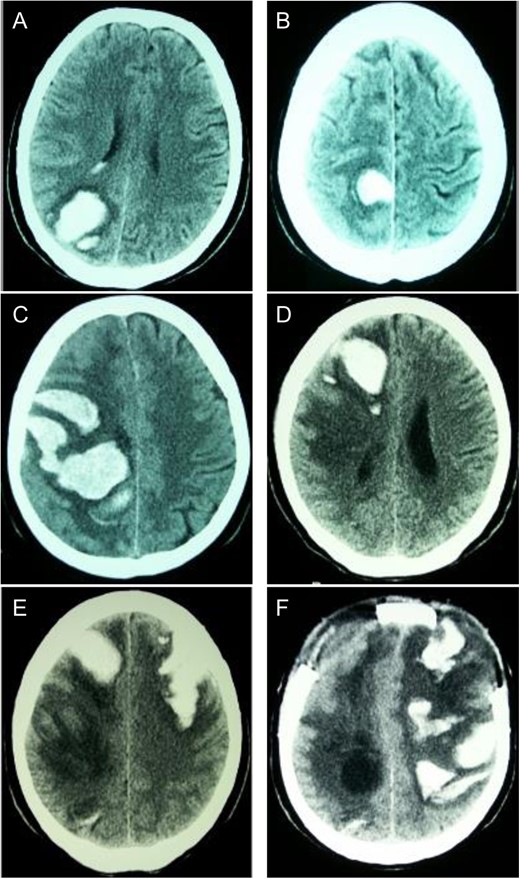
Sequential CT head scans. (A) 3.5 cm ICH in right parieto-occipital lobe with peripheral rim of edema and intraventricular extension; (B) 2.5 cm ICH anterior to first bleed with small ring of surrounding edema; (C) 7 × 4 cm right fronto-parietal ICH with intraventricular extension and subfalcine herniation; (D) 2 × 4 cm ICH in right frontal lobe with surrounding halo of edema and 8 mm subfalcine herniation; (E) 5 × 3 cm left anterior frontal lobe ICH with mild surrounding edema; (F) left fronto-parietal ICH with 2 cm midline-shift.
Second ICH: Five weeks after initial presentation the patient returned with a spontaneous onset of mild left leg weakness. Repeat CT revealed a new ICH anterior to the initial ICH, though the patient was neurologically stable (Fig. 1B).
Third ICH: Six weeks after initial presentation, the patient awoke with left sided hemiparesis, facial drooping and severe headache. Progressive decreased level of consciousness led to intubation in the ER and CT scan showed new ICH in the right fronto-parietal area (Fig. 1C). On examination, BP was 135/76, brainstem reflexes were intact and the patient localized only with her right upper extremity. Decerebrate posturing on the left side was demonstrated with brisk deep tendon reflexes and a positive left Babinski response. Routine hematological investigations were unremarkable. Urgent decompressive craniotomy was performed for evacuation of ICH.
Fourth ICH: Three weeks later, while in the Step-down Unit, the patient experienced another ICH localized in the anterior right frontal lobe (Fig. 1D).
Fifth ICH: Six days later, the patient experienced a sudden decreased level of consciousness with two generalized seizures. Repeat CT scan revealed a new ICH in the left anterior frontal lobe (Fig. 1D). Urgent craniotomy was performed with evacuation of bi-frontal hematomas and a brain biopsy specimen was sent for pathology analysis. Histopathological examination demonstrated no evidence of vascular malformation or neoplastic process. However, immunohistochemistry and Congo red staining (previously described [3]) confirmed beta-amyloid deposition in cortical and leptomeningeal vessel walls, and the adjacent brain parenchyma presented neuritic Alzheimer-type plaques (Fig. 2). Finally, blood specimen analysis via polymerase chain reaction/restrictive fragment length polymorphism revealed an ApoE genotype Є2/Є3.
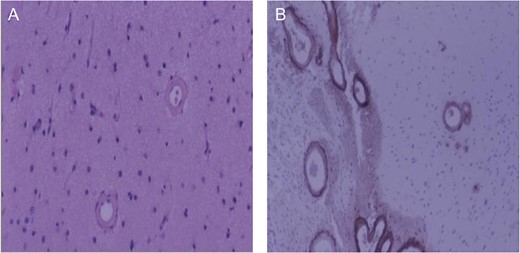
Brain biopsy of patient. (A) Low magnification, hematoxylin and eosin stain of cerebral cortex. Typical smooth vessel wall thickening associated with amyloid angiopathy; (B) Low magnification, beta-amyloid immunohistochemistry. Intense beta-amyloid deposition in leptomeningeal (left) and cortical (right) vessels.
Sixth ICH: Two weeks later, the patient’s neurological status deteriorated significantly and CT revealed a new left fronto-parietal ICH (Fig. 1F). In view of the likelihood of poor quality of life, a decision was made with the family to withdraw active care and the patient passed away shortly thereafter.
DISCUSSION
Despite previous recognition of the ApoE Є2 and Є4 genotype’s role in CAA-associated ICH, to the best of our knowledge the present case is novel in clinical presentation, investigation and management approach. Most notably, the patient’s six recurrent lobar ICHs—all at different times—were localized to multiple cerebral sites.
The genotype of our patient was Є2/Є3, indicating heterozygosity for the ApoE gene. These findings support the hypothesis of two distinct CAA pathologies. However, the paucity of literature discussing CAA-associated ICHs in patients expressing the ApoE Є2 allele is concerning and warrants further investigation for this population [3]. As highlighted by the present case, consideration of one’s genetic disposition can help facilitate the clinical prognostication and management of cerebrovascular risk factors [1].
ICH results in death or severe disability in more than half of patients—the worst prognosis of any cerebrovascular event reported [3]. There is a clearly demonstrated and known risk of CAA-associated ICH in ApoE Є2 and/or Є4 carriers, and the best predictor of clinical outcome in spontaneous CAA-associated ICH is hematoma volume at presentation [1, 2, 4]. Possession of the ApoE Є2 allele predisposes patients with lobar ICH to greater hematoma volume on presentation as well as increased risk for hematoma expansion, compared to ApoE Є4 allele expression [2]. Thus, the population expressing the ApoE Є2 genotype, as revealed in our patient, are at increased risk of ICH as well as recurrent ICH and mortality [1].
The ApoE Є2 genotype increases risk for recurrent macro and micro ICH as detected by magnetic resonance imaging (MRI) [4]. Baseline MRI following a major ICH in individuals possessing the ApoE Є2 genotype were found to have increased levels of micro-hemorrhages and increased risk of severe vessel damage near a hemorrhage site, which could forebode future ICH events [1, 4]. As such, screening MRI for ApoE Є2 expressers can potentially predict the risk of future ICH [1, 4]. Although, MRI could have aided with diagnosis of the present case, a negative cerebral angiogram conducted on a patient of this age who already experienced a lobar hemorrhage strongly suggested CAA, later confirmed via operative pathology.
Though craniotomy for ICH clot evacuation has been demonstrated to be effective, it must be recognized that recurrent hemorrhages associated with ApoE Є2 represent poor prognosis [1]. Given the increased risk of severe, recurrent ICHs within this population, further investigations into anticoagulant and thromboembolic preventive measures to increase prophylactic screening and further examinations of surgical hematoma evacuation results are necessary.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No sources of funding to declare.



