-
PDF
- Split View
-
Views
-
Cite
Cite
Ankur Sidhu, Su Kah Goh, Eunice Lee, Christopher Christophi, Marcos Perini, Salmonella typhimurium: a rare cause of mesh-related infection, Journal of Surgical Case Reports, Volume 2017, Issue 10, October 2017, rjx196, https://doi.org/10.1093/jscr/rjx196
Close - Share Icon Share
Abstract
The use of mesh in the management of abdominal wall hernias has significantly reduced the incidences of hernia recurrences. The placement of synthetic meshes to reinforce the abdominal wall is not without caveats. Synthetic meshes are associated with a risk of infection. Common causative microorganisms for mesh-related infection range from a diversity of gram positive, gram negative and anaerobic bacteria. However, non-typhoidal Salmonella spp. mesh-related infection remains poorly described in the literature. In this case, we report the management of an immunocompromised patient who developed Salmonella typhimurium mesh-related infection that was complicated by abscess formation.
INTRODUCTION
The use of mesh in the management of abdominal wall hernias has led to a significant decrease in hernia recurrences. Despite sterile operative techniques and perioperative prophylactic antibiotics, the use of synthetic mesh is associated with a small risk of infection [1].
The usual causative organisms associated with cases of mesh-related infection are Staphylococcus spp., Streptococcus spp., gram negative bacteria (mainly Enterobacteriaceae spp.) and anaerobeic bacteria (including Peptostreptococcus spp.) [2]. However, non-typhoidal Salmonella spp. mesh-related infection is poorly described in the literature.
Non-typhoidal Salmonella species are important food borne pathogens. Gastroenteritis is the most common clinical presentation of non-typhoidal Salmonella infection and up to 5% of individuals will develop bacteraemia [3]. Abscess formation in individuals with non-typhoidal Salmonella spp. infection is rare, especially in immunocompetent hosts and in the modern era of antibiotics [4].
Herein, we report the management of an immunocompromised patient who developed Salmonella typhimurium mesh-related infection that was complicated by abscess formation on a background of a recent episode of acute gastroenteritis.
CASE REPORT
A 59-year-old man presented to the emergency department with a 5-day history of worsening left iliac fossa pain, fevers and vomiting. He had a history of chronic lymphocytic leukaemia (currently in remission), diverticular disease, laparoscopic splenectomy for idiopathic thrombocytic purpura, laparoscopic cholecystectomy and laparoscopic intraperitoneal mesh (C-QUR™ Mesh; Atrium, USA) repair of a port site hernia (6 months following the cholecystectomy).
He described an episode of acute gastroenteritis 2 weeks prior to this presentation. His symptoms promptly resolved without any medical intervention and his bowel function was reportedly normal. He denied any urinary symptoms, respiratory or coryzal symptoms. He did not have any loss of weight or any night sweats.
On arrival to emergency department, he was febrile with a temperature of 38.2°C. His vital signs were normal. On abdominal examination, he had mild tenderness in his left iliac fossa without any evidence of peritonism. A vague and heterogeneous mass was palpable in the area of tenderness.
His blood tests showed a white cell count of 18.9 × 109/L with neutrophils of 8.7 × 109/L and lymphocytes of 7.4 × 109/L. The renal function was normal but liver function tests were mildly deranged: ALT 222 U/L, AST 118 U/L, GGT 175 U/L, ALP 247 U/L and bilirubin of 20 μmol/L. The C-reactive protein was 150 mg/L. The patient underwent a computer tomography (CT) scan of his abdomen and pelvis which showed a 6 cm × 7.5 cm × 2.5 cm collection consistent with an abscess underlying the anterior abdominal wall in the region of left iliac fossa (Fig. 1). This in close proximity to the mesh used in his previous hernia repair that was performed earlier. There was evidence of uncomplicated sigmoid diverticular disease.
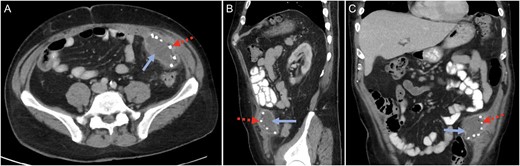
Computed tomography (CT) scan in the (A) cross-sectional, (B) sagittal and (C) coronal planes confirming a mesh-related abscess in the left iliac fossa. Solid blue arrow marks the abscess. Broken red arrow marks the metallic tack that was used to secure the intraperitoneal mesh.
He underwent ultrasound-guided aspiration of the collection and S. typhimurium phage type 135 was cultured. The microorganism was sensitive to ampicillin, ceftriaxone, ciprofloxacin, cotrimoxazole with the highest susceptibility (minimum inhibition concentration) to ciprofloxacin of 0.032 mg/L. The identical strain was also cultured from his faecal specimen. His blood cultures were negative. He was treated with 2 g of intravenous ceftriaxone daily.
Given the abscess was related to the previous mesh repair, he underwent open drainage, washout and debridement of the abscess and removal of the infected mesh. The abdominal fascia was primarily repaired using simple interrupted 1/0 prolene sutures. His post-operative course was uneventful.
The antibiotic regime was changed to oral ciprofloxacin upon discussion with the infectious disease physicians. He was discharged home 4 days later and remains well 6 months after discharge.
DISCUSSION
The use of mesh for elective incisional hernia repair has become increasingly common. For incisional hernias, mesh can be placed anterior to the fascia (on-lay technique), posterior to the fascia and the muscular layers (sub-lay technique), and directly beneath the peritoneum (intraperitoneal technique). But the use of mesh is not without caveats. Mesh-related complications include infection, adhesions, migration, bowel obstruction and enterocutaneous fistula. There are a variety of meshes available with different properties with a view to decrease mesh-related complications. However, mesh is still a foreign material to which bacterial seeding can occur. The management of infected mesh is challenging and almost always, requires surgical debridement and mesh removal.
Salmonella is an enteroinvasive gram-negative bacterium and a primarily enteric pathogen. Salmonella spp. infections in humans can be classified into five clinical groups: enteric fever, septicaemia without localisation, focal disease, gastroenteritis and the chronic carrier state [5]. Focal distant disease can occur following overt or occult bacteraemia (i.e. seeding of bacteria onto distant sites) [6]. Extremes of ages, immunosuppression, underlying malignancy, intravenous drug use and previous trauma have been identified as risk factors for Salmonella spp. infections. Despite several published reports in the literature of Salmonella spp. infections, mesh-related abscesses related to S. typhimurium has not been described [6–9].
In this case, the patient developed an episode of acute gastroenteritis 2 weeks prior to his presentation. This episode likely represents the initial inoculation of S. typhimurium. The course of Salmonella spp. gastroenteritis is generally mild and self-limiting and this was consistent with his described symptoms.
Given his immunocompromised background of asplenia and chronic lymphocytic lymphoma, it is likely that he developed transient bacteraemia and subsequently haematogenous seeding of the bacteria onto the mesh. The possibility of intestinal translocation of the bacteria leading to the infection of the intraperitoneal mesh was considered but this was difficult to definitively ascertain.
A CT scan was performed to establish the diagnosis in this patient. Once the diagnosis of mesh-related infection complicated by an abscess formation, he was treated with antibiotics, surgical washout and debridement, and surgical removal of mesh. It was considered that prompt diagnosis and treatment of the mesh-related abscess was central towards an uneventful post-operative course, especially in this immunocompromised patient.
To our knowledge, this is the first reported case of mesh-related abscess due to S. typhimurium infection. Although rare, this case highlights that Salmonella spp. bacteraemia can lead to abscess formation in at risk patients.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
Author notes
Co-first author.



