-
PDF
- Split View
-
Views
-
Cite
Cite
Alec F. Snow, Milena Vannahme, Laura Kettley, Anne Pullyblank, Ruptured hepatic artery aneurysm precipitated by gangrenous perforated appendicitis: a case report, Journal of Surgical Case Reports, Volume 2016, Issue 5, May 2016, rjw083, https://doi.org/10.1093/jscr/rjw083
Close - Share Icon Share
Abstract
Hepatic artery aneurysms are among the most common visceral artery aneurysms although still relatively rare. Repair of aneurysms >2 cm in diameter is important due to the high rate of rupture and associated mortality. Here, we present a case of a sudden rupture of a hepatic artery aneurysm after presentation with a perforated gangrenous appendicitis. There is increasing evidence that expansion and rupture of abdominal aortic aneurysm is related to degradation of elastin and collagen by matrix metalloproteinases (MMPs). Elastin degradation leads to expansion, while collagen degradation leads to rupture. The activity of MMPs has been shown to be upregulated by both sepsis and peritonitis. Here, we suggest that the inflammation from sepsis and peritonitis led to the activation and/or upregulation of MMPs, which precipitated aneurysm rupture via collagenase activity.
INTRODUCTION
Hepatic artery aneurysms are among the most common types of visceral aneurysms, accounting for 20% of the total [1]. Recognized etiological factors include atherosclerosis, medial degeneration, portal hypertension, trauma, infection, congenital defects, vasculitis and iatrogenic causes. Epigastric and right upper quadrant pain is the most common presentation, although many are found incidentally on routine imaging. The classic Quinke’s triad of gastrointestinal haemorrhage, biliary colic and obstructive jaundice is seen in <30% of presentations [2].
Spontaneous rupture of hepatic aneurysms is associated with a high mortality rate of up to 40%; hence, elective repair is generally recommended in aneurysms >2 cm in size [3].
The cause of abdominal aortic aneurysm (AAA) expansion and rupture is an area of ongoing research. The gradual elucidation of the biological processes underlying aneurysm rupture is changing the view that mechanical forces are solely responsible for aneurysm evolution.
CASE REPORT
An 80-year-old Caucasian man presented as an emergency with a 7-day history of central abdominal pain that had moved to the right iliac fossa (RIF) and increased in severity over the last 2 days. He was anorexic and nauseated with no diarrhoea or vomiting. His observations were blood pressure 112/86 mmHg, pulse 90/minute, respiratory rate 18/minute, oxygen saturations 98% on air and temperature 35.9°C. His abdomen was distended, and he had signs of localized peritonism in the RIF. His blood tests showed a raised C-reactive protein of 35 mg/L, white cell count of 14.1 × 109/L and haemoglobin of 14.2 g/dL. His admission chest radiograph was unremarkable. A diagnosis of acute appendicitis was made, and the patient was booked at 0100 for a laparoscopy and appendicectomy on the emergency list the following morning.
The patient was stable overnight but suddenly deteriorated on the ward at 0800. He became haemodynamically compromised with a blood pressure of 85/60 mmHg, pulse of 135/minute, saturations of 95% on air and a respiratory rate of 35/minute. Lactate on arterial blood gas was 4.5 mmol/L. A diagnosis of septic shock was made, and he was resuscitated with intravenous fluids and antibiotics before immediate transfer to the theatre for an emergency laparotomy.
On opening the abdomen, there was a large volume of intra-peritoneal blood but also localized faecal contamination in the RIF due to a necrotic, perforated appendix. The bleeding was localized to the lesser sac in the right upper quadrant. The patient’s abdomen was packed and the appendicectomy completed. As the source of the bleeding was unclear, the patient was transferred for an emergency computed tomographic angiogram without prior closure of his abdomen. This showed a leaking saccular common hepatic artery aneurysm measuring 25–30 mm in diameter. Prompt coil embolization was arranged using a transfemoral approach until no bleeding was evident after 5 minutes of observation. On return to the theatre for abdominal closure, haemorrhage was noted after removal of packs surrounding the aneurysm and surgical repair of the aneurysm was required. The patient required a total of 11 units of packed red cells. He made a slow but uneventful recovery and was discharged 19 days later (Figs 1 and 2).
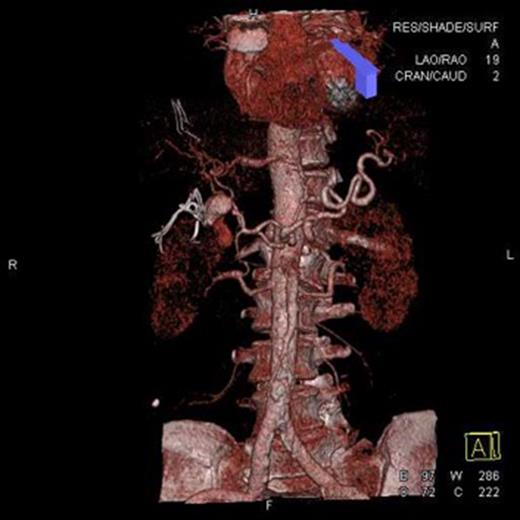
Computed tomographic angiogram. Three-dimentional reconstruction of hepatic vascular tree showing a 30-mm saccular aneurysm of the hepatic artery.
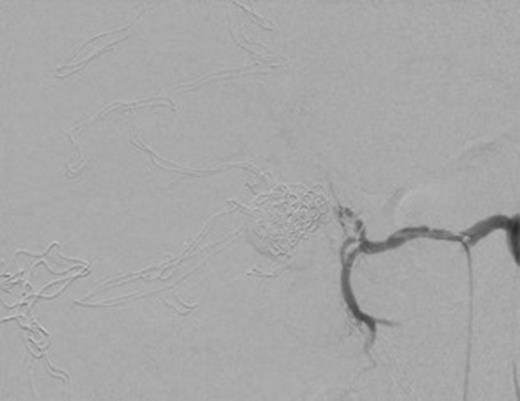
Post coil embolization arteriogram with 5-minute delay showing coils inside aneurysm and no extravasation of contrast into aneurysmal sac.
DISCUSSION
Aneurysms can rupture at any size suggesting that the traditional solely mechanical explanation for rupture is insufficient. It has been suggested that AAA expansion and rupture is related to increased enzymatic activity at the aneurysm wall. Specifically, there is increased proteolysis due to elevated concentration and activity of matrix metalloproteinases (MMPs) [4]. MMPs are a group of 23 zinc-dependent cell surface proteases that act upon extracellular matrix components, including elastin and collagen, which are found in abundance in the arterial wall [5, 6]. The same pathological processes behind degenerative aneurysms have been reported in aneurysms at different sites inferring that research findings from one type of aneurysm can be related to those at other sites [7].
As an AAA expands, elastin content decreases, and in response to the increased wall stretch collagen concentration is elevated. Elastin is thought to be responsible for load bearing at physiological pressures, while collagen takes over this function at higher pressures [4]. The degradation of elastin leads to aneurysm dilatation, while the degradation of collagen leads to rupture [8]. Prior to rupture, AAAs contain a predominance of collagen Types I and III. End-stage aneurysms are composed of extensive inflammatory infiltration ultimately resulting in the release of cytokines that produce and activate proteolytic enzymes including MMPs [4, 9]. MMP-8 and MMP-9 have both been implicated in AAA rupture. Both are known to have collagenase activity, and their elevation is well described in AAA disease and may impair mechanical stability. MMP-8 is a potent Type 1 collagenase, while MMP-9 acts on partially degraded fibrillar collagen fragments and elastin [6, 10].
Interestingly for this case, MMPs have been shown to be involved in both peritonitis and sepsis. Serum MMP-8 and MMP-9 levels are significantly higher in patients with severe sepsis than in healthy controls, suggesting that they may contribute to the host response during sepsis. These specific MMPs are released from neutrophils and activated in sepsis; indeed, high levels of MMP-8 may be associated with increased mortality. Additionally, peritoneal mesothelial cells produce MMPs and levels and activity of MMP-9 are both raised in peritoneal fluid during active peritonitis [6]. It is a source of controversy whether distant injury can specifically increase aortic collagenase activity and more research is required to support this, although cytokines are well known to mediate systemic effects. Prior to aneurysm rupture, there were no significant haemodynamic changes that may have predisposed to this event.
This case suggests that the rupture of pre-existing aneurysms can be precipitated by systemic sepsis or peritonitis. No previous reports highlight this as a direct risk factor. The precise mechanism is unclear, but review of the literature suggests that this may be due to induction of an inflammatory response that can up-regulate collagenase activity at distant sites [4]. This relationship between inflammation and aneurysm rupture has significance for patients with active inflammatory conditions and known aneurysmal disease.
Antibiotics from the tetracycline group of antibiotics chemically inhibit MMPs and doxycycline has been shown to be a non-specific MMP inhibitor capable of stabilizing aneurysm size, while statins down-regulate the production of MMP-9 in AAAs [5]. Further research is needed into the rate of rupture of aneurysms in patients with an active inflammatory process as well as the role of MMPs in aneurysmal disease in order that targeted therapies can be produced to down-regulate MMP activity.
CONFLICT OF INTEREST STATEMENT
None declared.



