-
PDF
- Split View
-
Views
-
Cite
Cite
Hasib Ahmadzai, Ali Khalil, Ruth A. Mitchell, Bernard Kwok, An unusual case of spinal cord compression from concomitant spinal epidural lipomatosis and Hodgkin's lymphoma, Journal of Surgical Case Reports, Volume 2016, Issue 3, March 2016, rjw025, https://doi.org/10.1093/jscr/rjw025
Close - Share Icon Share
Abstract
Spinal epidural lipomatosis (SEL) results from an abnormal accumulation of unencapsulated fat within the epidural space and is a rare cause of spinal cord compression, which needs to be considered with a high index of suspicion. It most commonly occurs secondary to chronic corticosteroid use and endocrinopathies. Idiopathic cases are highly associated with obesity. We report an unusual case of idiopathic thoracic SEL in a 69-year-old male, with an adjacent infiltrative Hodgkin's lymphoma and associated vertebral crush fracture, which resulted in ataxia and sensory loss. Magnetic resonance imaging scans displayed extensive SEL and an infiltrative disease process causing thoracic cord compression. Surgical decompression confirmed the presence of extensive epidural lipomatosis and Hodgkin's lymphoma and subsequently led to improvement in neurological symptoms. To our knowledge, this is the first reported case of concomitant SEL with an adjacent Hodgkin's lymphoma resulting in cord compression.
INTRODUCTION
Spinal epidural lipomatosis (SEL) is a rare condition, manifested by increased deposition of adipose tissue in the spinal epidural space [1]. Severe cases can cause compression of the neural elements [2, 3]. Important aetiological factors include exogenous or endogenous hypercortisolism, hypothyroidism, hyperprolactinaemia, adrenal tumours and other endocrinopathies. There are many cases of unknown aetiology, although obesity has a strong association with idiopathic cases [1]. We report an unusual case of spinal cord compression from thoracic SEL and associated Hodgkin's lymphoma with a vertebral crush fracture.
CASE REPORT
A 69-year-old man with a body mass index of 26.5 kg/m2 presented with a 10-day history of ataxia and reduced sensation in both lower limbs and trunk. He denied any history of bowel or bladder disturbance. Upper and lower limb neurological examination was otherwise unremarkable except for upgoing plantar responses bilaterally, reduced light touch sensation bilaterally in his lower limbs up to his trunk with a T5 sensory level and an ataxic gait.
The patient's past medical history included essential hypertension and type 2 diabetes mellitus treated with metformin. Mid-thoracic back pain had also been an active medical problem, which had been under investigation for over 1 year, following a fall. A full blood count had identified lymphopenia and a normocytic, normochromic anaemia. Electrolytes and creatinine, liver function tests, thyroid function tests, vitamin B12, folate, adrenocorticotrophic hormone (ACTH) and cortisol levels were all normal. A computed tomography (CT) scan of his thoracic spine revealed T5 and T6 vertebral crush fractures, with 30–40% loss of height of T6, for which he had been on calcium, vitamin D supplements and zoledronic acid injections. He had not been taking corticosteroid medications and denied previous steroid use. The T5 and T6 thoracic fractures had the appearance of underlying mixed lytic and sclerotic lesions suggestive of neoplasia. A nuclear medicine bone scan highlighted the thoracic lesions but did not identify other sources of abnormal tracer uptake (Fig. 1A and B). A CT scan of the chest, abdomen and pelvis did not detect any other pathology. A T6 vertebral bone biopsy had also been taken a few months prior to this presentation, which only showed reactive bone and cartilaginous fragments with no tumour cells isolated. Tumour markers were negative apart from a β2-microglobulin level of 3.1 mg/l (reference range 0.7–1.8 mg/l).
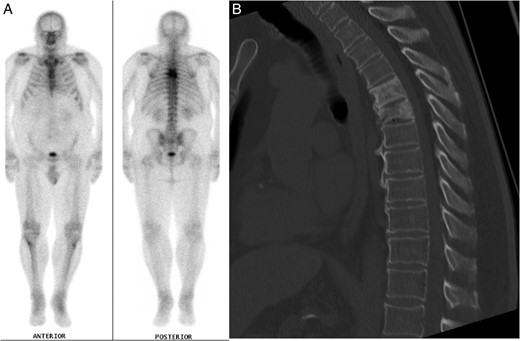
(A) Nuclear medicine bone scan demonstrating tracer uptake in the T5 and T6 vertebral fractures with no evidence of other abnormal uptake. (B) Mid-sagittal CT scan of the thoracic spine during clinical presentation with ataxia, displaying the vertebral crush fractures and SEL.
Investigation for his recent ataxia and sensory changes included CT of his thoracic spine (Fig. 1B) and whole spine magnetic resonance imaging (MRI) scan (Fig. 2), which demonstrated marrow infiltrative disease involving the T6 and T5 vertebra, corresponding to the findings seen on previous imaging. The appearance of the T6 fracture compared with CT imaging from 6 months previously had remained largely unchanged. However, there was a significant paravertebral (especially left epidural) disease at T6, likely related to an underlying infiltrative lesion resulting in compression of the thecal sac without spinal cord oedema (Fig. 2). Interestingly, superimposed dorsal epidural lipomatosis was also seen extending along the cervicothoracic spinal canal, from C7 to T10, most severe at the T5 and T6 levels, further contributing to canal stenosis and likely causing the patient's symptoms.
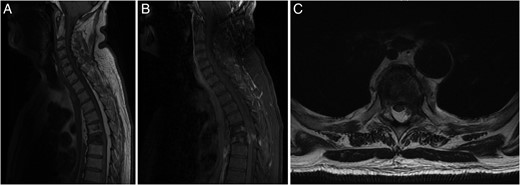
(A) Sagittal T1-weighted MRI showing the T5 and T6 vertebral compression fractures with extensive hyperintense SEL from C7 to T10. (B) Sagittal FAT SAT T1-weighted MRI following gadolinium contrast showing contrast enhancing paravertebral disease mainly at T6 level, with enhancing epidural disease with further canal stenosis from epidural lipomatosis. (C) Axial T2-weighted MRI displaying through T6 level showing compression and right lateral displacement of the thecal sac and spinal cord.
A T6 thoracic laminectomy with T3–T5 and T7–T8 laminoplasty was performed for decompression of the spinal cord and removal of lipomatosis. A piece of fatty tissue measuring 120 × 15 × 10 mm was removed from the T6 spinal canal level (Fig. 3). Histopathological analysis of the fatty tissue was consistent with lipoma. Other spinal canal tissue revealed scattered Reed–Sternberg cells with prominent nucleoli and immunohistochemistry features diagnostic for Hodgkin's lymphoma.
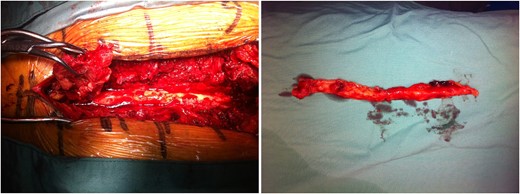
Intraoperative images displaying extensive epidural lipomatosis of the thoracic spine.
The patient had an uneventful post-operative recovery prior to further chemotherapy treatment. He remained well at follow-up with significant improvement of his neurological symptoms.
DISCUSSION
To our knowledge, this is the first reported case of spinal cord compression secondary to idiopathic SEL with an adjacent, concomitant vertebral crush fracture caused by infiltrative Hodgkin's lymphoma. Our patient had not previously taken corticosteroid medications, as a diagnosis of lymphoma had not been made prior to surgery. SEL has been described in patients with non-Hodgkin's lymphoma [4, 5] and other haematological malignancies [4, 6–9] who had been taking exogenous corticosteroids, patients on long-term steroid treatment, as well as carcinoid-induced SEL caused by ACTH secreting tumours with Cushing's syndrome [2]. To our knowledge, this is the second case reported of SEL in a patient with Hodgkin's lymphoma; with one previous case in a patient with only inguinal lymph node involvement [7].
It is believed that with progressive epidural fat accumulation, a critical threshold is reached whereby the epidural space-occupying lesion causes impairment of neurological function [3]. It was interesting that our patient had not been on corticosteroids prior to presentation as a diagnosis of lymphoma had not been previously made with negative bone biopsy results. A review of SEL found that idiopathic SEL commonly affects the lumbosacral spine, while secondary causes including cases associated with neoplasia are seen more frequently in the thoracic spine [1, 10].
When SEL is asymptomatic or presents with slowly progressive symptoms, conservative management to treat risk factors such as weight loss and weaning of corticosteroid medications may be appropriate. With acute neurological compromise—as in our patient—decompressive surgical intervention is warranted, with adipose tissue resection [3]. A previous systematic review showed that 88% of patients reviewed underwent surgical decompression, with a 60% rate of full neurological recovery in all cases of SEL, and 50% having full recovery in cases of idiopathic thoracic SEL [1].
Although a rare condition, a high index of clinical suspicion is required to consider SEL as a cause of spinal cord compression. It was more unusual to identify adjacent Hodgkin's lymphoma also contributing to canal stenosis. Early MRI imaging is essential for diagnosis, with particular focus on T1-weighted imaging and early consideration of surgical decompression in patients with neurological deterioration which can potentially correct neurological deficits.
CONFLICT OF INTEREST STATEMENT
None declared.



