-
PDF
- Split View
-
Views
-
Cite
Cite
Keyur Parikh, Leena Khaitan, Radiofrequency ablation coupled with Roux-en-Y gastric bypass: a treatment option for morbidly obese patients with Barrett's esophagus, Journal of Surgical Case Reports, Volume 2016, Issue 3, March 2016, rjw007, https://doi.org/10.1093/jscr/rjw007
Close - Share Icon Share
Abstract
Barrett's esophagus (BE) is a premalignant condition that is associated with the development of esophageal adenocarcinoma. Risk factors that have been associated with the development of BE include male gender, Caucasian race, chronic gastroesophageal reflux disease, smoking, age >50 and obesity. The current management of BE is dependent on underlying pathological changes and treatment can range from surveillance endoscopy with daily proton pump inhibitor (PPI) therapy in the setting of intestinal metaplasia or low-grade dysplasia (LGD) to radiofrequency ablation (RFA), endoscopic mucosal resection or surgical resection in the setting of high-grade dysplasia. We report the case of a morbidly obese patient who was found to have long-segment BE with LGD during preoperative work-up for weight loss surgery with Roux-en-Y gastric bypass (RYGBP). The patient underwent successful RFA for the treatment of her BE before and after her RYGBP procedure. At 5-year follow-up, there was minimal progression of BE after treatment.
INTRODUCTION
Barrett's esophagus (BE), the condition in which metaplastic columnar mucosa relays a predisposition to esophageal adenocarcinoma (EAC) by replacing existing squamous mucosa, has an estimated incidence to be nearly 5.6% in the general US population [1]. The incidence rate of EAC has increased 6-fold over the last three decades, while rates of colorectal cancer, breast cancer and lung cancer have remained stable [2]. A recent study by Thrift et al. [3] found that increasing body mass index (BMI) increases this baseline risk of developing BE by an additional 12% per kg/m2. Among the identified risk factors for the development of BE including Caucasian race, male gender, inherited germline mutations, chronic gastroesophageal reflux disease (GERD) and obesity, only the latter two are potentially modifiable. A variety of treatment options have been proposed including fundoplication, PPIs, radiofrequency ablation (RFA), endoscopic mucosal resection and even esophagectomy. Interestingly, there is a paucity of data regarding the course of BE after bariatric surgery.
We encountered a morbidly obese individual who was being evaluated for weight loss surgery and was found to have long-segment BE on preoperative endoscopic evaluation. We implemented a unique treatment algorithm using a combination of Roux-en-Y gastric bypass (RYGBP) and RFA resulting in successful management of BE.
CASE REPORT
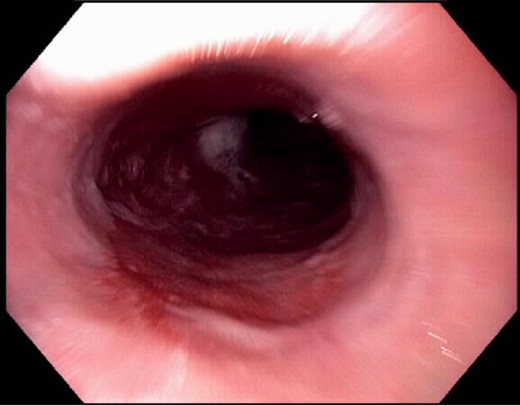
A 47-year-old morbidly obese male with a BMI of 41 kg/m2 presented in the clinic regarding evaluation for weight loss surgery. He reported symptoms consistent with chronic GERD and a remote history of BE; however, he had not undergone prior surveillance endoscopy and he did not recall taking a PPI. In addition, he also reported that multiple family members also had a history of BE. A preoperative esophagogastroduodenoscopy (EGD) was performed and revealed an 8-cm segment of circumferential BE (Fig. 1). The histopathology examination revealed intestinal metaplasia consistent with BE with evidence of low-grade dysplasia. Based on these findings, the decision was made to proceed with a combined management strategy consisting of RFA and RYGBP.
Prior to his RYGBP, he underwent repeat EGD with RFA using the HALO360 System (BÂRRX Medical, Sunnyvale, CA, USA) to ablate his BE using energy settings of 300 W and 10–12 J/cm2. Immediately after his ablation procedure, he was started on a twice-daily PPI and sucralfate. Six weeks later, the patient underwent a laparoscopic RYGBP procedure, in which a 30 cm2 gastric pouch and a 150-cm Roux limb were created. Repeat endoscopy was performed during the surgery for leak test and presence of BE was, once again, noted. No intraoperative interventions were performed for this BE. The remainder of his postoperative course was uneventful.
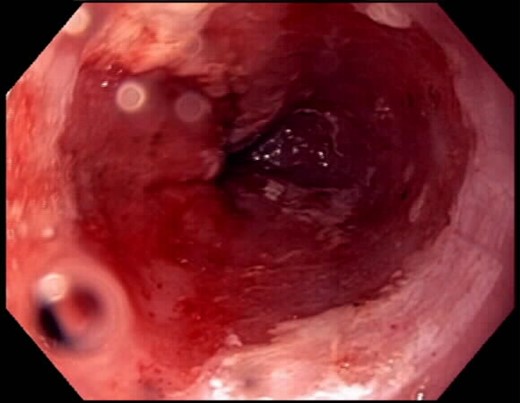
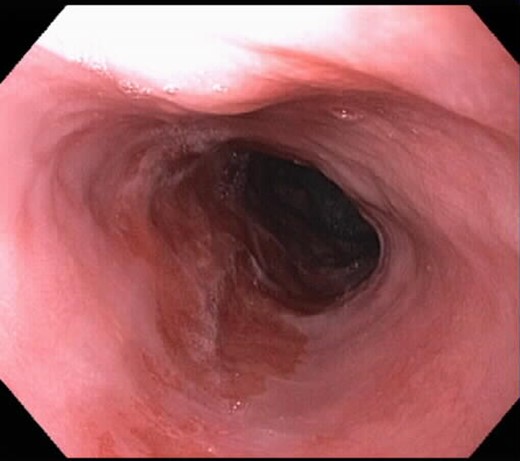
After his RYGBP, the patient was continued on 40 mg of a PPI daily instead of the twice-daily dose because the anatomical changes from surgery are known to reduce esophageal acid exposure. He underwent repeat EGD ∼14 weeks after surgery, which is a total of 5 months after his initial ablation, and there was no progression in the size of the BE segment at 8 cm. Given the lack of progression, we chose to ablate the patient a second time, but did not conduct any biopsies. The post-procedural image after the second ablation is shown in Fig. 2. During surveillance EGD, which was performed at a 4-month interval (9 months after initial diagnosis of BE), only a 2-cm circumferential segment of BE was seen with a proximal island 5 cm from the gastroesophageal junction, thereby indicating a significant reduction of BE segment from the original time of diagnosis (Fig. 3). A third ablation was performed during this EGD using the Halo90 System (BÂRRX Medical). Again, no biopsies were conducted as there was macroscopic evidence of resolving BE. The patient has completed ongoing surveillance endoscopy demonstrating minimal progression of the Barrett's over time.
DISCUSSION
The rise in the incidence of BE and EAC mirrors the obesity epidemic in western countries. A retrospective cross-sectional study from veterans association in the USA demonstrated that obesity is associated with a two-and-half-fold increase in the risk of BE [4]. They found that for each 10-pound increase in (4.54 kg) weight or five-point increase in BMI, there was a 10 and 35% increase in the risk of BE, respectively. Central adiposity in this study was independently associated with Barrett's when controlling for BMI, suggesting that central obesity, and not only BMI, was the link with BE [4].
The American College of Gastroenterology guidelines for the management of BE recommend endoscopic surveillance, RFA or, in certain cases, esophagectomy, depending on the degree of dysplasia [5]. But, the clear association of obesity with Barrett's mucosa mandates treating both morbid obesity and GERD/Barrett's. Thus, we chose to treat above-mentioned case with a sandwich regime consisting of RFA and gastric bypass.
The case in the current study had long-segment Barrett's and low-grade dysplasia, so we targeted the dysplasia with RFA followed by gastric bypass to treat morbid obesity and eliminate acid exposure.
Gastric bypass in patients with BE and morbid obesity is an excellent antireflux operation, proved by the disappearance of symptoms and the healing of endoscopic esophagitis or peptic ulcer in all patients, which is followed by an important regression to cardiac mucosa that is length-dependent and time-dependent [6]. Cobey et al. demonstrated complete regression of Barrett's after 1 year of gastric bypass [7].
This case is unique in that we successfully used a combination approach of a RYGB ‘sandwiched’ by RFA to treat a patient with long-segment BE and morbid obesity. RFA of the esophageal mucosa for dysplasia has been gaining increasing popularity. It is performed as an outpatient procedure under conscious sedation. It is well tolerated by the patients with no or minimal morbidity. Photodynamic therapy (PDT) is the other modality reported to be effective for dysplasia [8]. However, the complications and morbidity associated with PDT (photosensitivity, need for isolation and strictures) are much more than with RFA. In a recent multi-institutional randomized study, complete eradication was seen in 90.5% of patients with low-grade dysplasia who were in the ablation group when compared with 22.7% patients who were in the sham group (P < 0.001) [9]. Similar results were reported from the study in patients with high-grade dysplasia. Overall, a complete eradication of Barrett's was seen in 77.4% in the ablation group when compared with 2.3% in the sham group.
In a study utilizing circumferential balloon-based ablation of non-dysplastic BE in 100 patients, a complete remission was seen in 70% of patients at 1-year follow-up [9]. We utilized the balloon-based circumferential RFA of the dysplastic mucosa for the initial treatments, but as the lesion size decreased we utilized more targeted ablation. This helps in tailoring the ablation according to the size of the dysplastic lesion. The patient tolerated every ablation session well with only complaint of mild pain for 1–2 days following the procedure. The patient had no strictures at 5-year follow-up.
This is a novel method of treating BE in patients with morbid obesity. On one hand, gastric bypass is doubly beneficial in eliminating acid exposure and weight loss, whereas the RFA is helpful in targeting the dysplastic mucosa.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- obesity
- smoking
- barrett's esophagus
- esophageal adenocarcinoma
- endoscopy
- gastroesophageal reflux disease
- follow-up
- male
- obesity, morbid
- precancerous conditions
- preoperative care
- european continental ancestry group
- proton pump inhibitors
- radiofrequency ablation
- surveillance, medical
- intestinal metaplasia
- bariatric surgery
- gastric bypass, roux-en-y
- excision
- endoscopic mucosal resection
- dysplasia



