-
PDF
- Split View
-
Views
-
Cite
Cite
Amro Al-Habib, Essam A. Elgamal, Saleh Aldhahri, Riyadh Alokaili, Rami AlShamrani, Abdulaziz Abobotain, Khulood AlRaddadi, Hisham Alkhalidi, Large primary leiomyoma causing progressive cervical deformity, Journal of Surgical Case Reports, Volume 2016, Issue 11, November 2016, rjw190, https://doi.org/10.1093/jscr/rjw190
Close - Share Icon Share
Abstract
Leiomyomas are benign smooth tumors that rarely affect the neck area. Complete surgical resection is the treatment of choice. Here, we describe a 13-year-old girl with a large leiomyoma of the neck, which increased in size after incomplete resection. The tumor caused progressive cervical kyphotic deformity, difficulty breathing and severe malnourishment. The tumor was resected successfully in a second surgery, and the patient is stable after 3 years of follow-up. Histopathologically, the tumor was consistent with leiomyoma and showed strong reactivity to specific smooth muscle markers, such as desmin and caldesmon. This is the second reported case demonstrating massive growth of a leiomyoma, with emphasis on complete resection from the beginning.
INTRODUCTION
Leiomyomas are benign nonepithelial tumors most commonly encountered in the uterus, gastrointestinal tract and skin [1, 2]. They are defined as circumscribed smooth muscle cell tumors according to the World Health Organization [2]. There are three main types of leiomyoma: solid, vascular (angioleiomyoma) and epithelioid (leiomyoblastoma) [1, 2]. All are well capsulated and show little cellular pleomorphism or mitotic activity, with the solid variant being the most common [2, 3]. Previous reports have shown that the lung is the most common site of metastasis of such tumors, followed by the mediastinum, nervous system, skin and bones [4]. However, some unusual reported sites of metastasis include the cranial epidural space and the right ventricle of the heart [4, 5].
Primary leiomyomas of the neck are extremely rare, most of which arise from the esophagus (0.3% of all esophageal leiomyomas). Only one previous report described an extraesophageal leiomyoma of the neck region [2]. Treatment of such tumors is primarily surgical, with no reported recurrences [1].
Here, we describe a young girl with a large leiomyoma of the neck, which increased in size after incomplete resection, causing significant cervical deformity. This case emphasizes the importance of complete surgical resection.
CASE REPORT
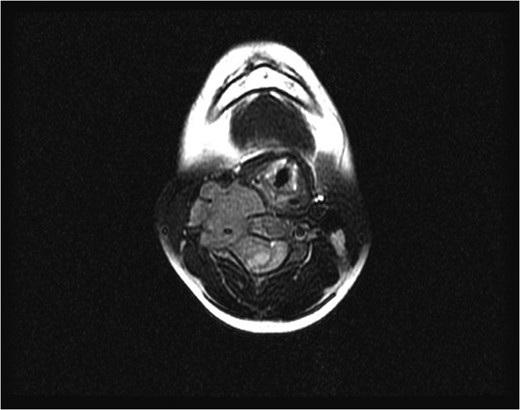
Initial axial magnetic resonance image, showing a tumor compressing the spinal cord and encasing the vertebral artery at the C4 level.
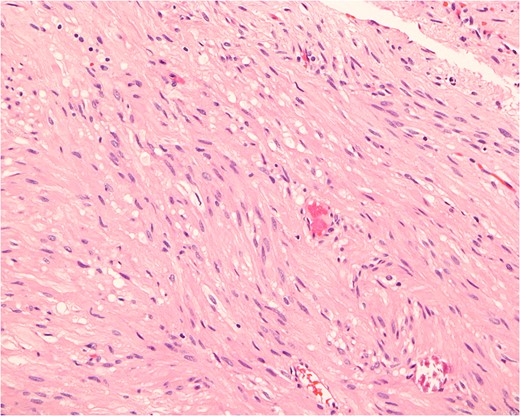
Histopathologically, the tumor consists of cells resembling normal smooth muscle cells. The cells are uniform, elongated and spindle-shaped, and exhibit a cigar-shaped nucleus (hematoxylin & eosin stain, magnification ×200).
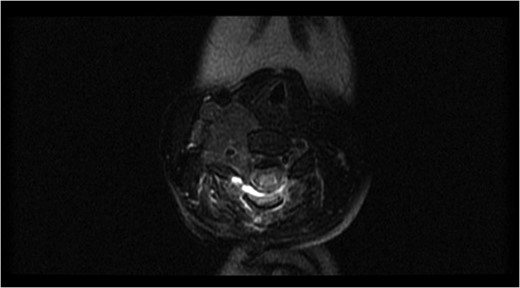
Axial magnetic resonance image after the first surgery, showing complete resection of the intraspinal component of the tumor, with a residual tumor and presence of a spinal cord signal at the C4 level.
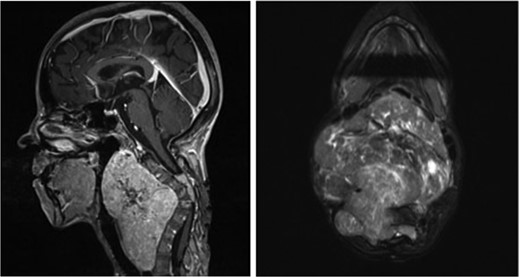
Sagittal (left) and axial (right) T1-weighted contrast-enhanced magnetic resonance images before the second surgery, showed increased size of the extraspinal component of the tumor, with kyphotic deformity of the cervical spine.
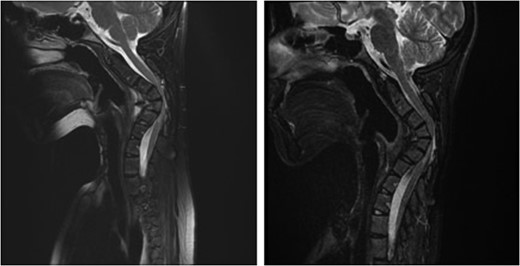
Sagittal magnetic resonance image 3 years after the second surgery, showing small soft tissue enhancement, suggesting a possible residual tumor, which remained unchanged with follow-up.
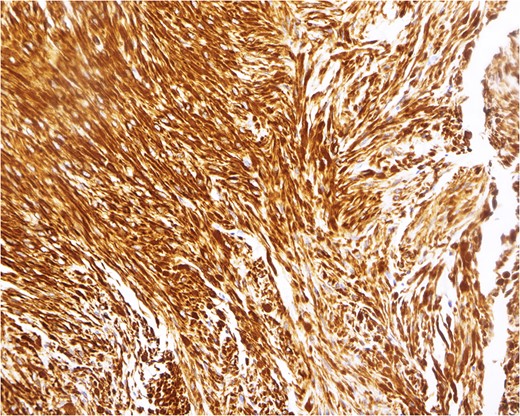
Immunohistochemical analysis, demonstrating a pattern consistent with leiomyoma. In this example, the tumor cells react strongly to a smooth muscle marker (desmin stain, magnification ×200).
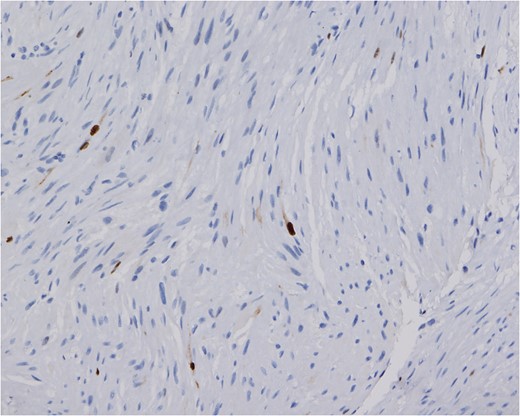
The Ki-67 proliferative index of the tumor is low. Only approximately 1% of the nuclei show reactivity (Ki-67 stain, magnification ×200).
DISCUSSION
Primary cervical leiomyoma with calcification and ossification is extremely rare, with only one case found in the literature [2]. The current case represents a unique tumor in its large size, progression and associated kyphotic deformity. Myofibroma/myofibromatosis could be a differential diagnosis of this patient's pathology. However, the diagnosis of leiomyoma was based on the morphologically apparent smooth muscle differentiation and minimal fibrous tissue element, with strong reactivity of the cells to specific smooth muscle markers, such as desmin and caldesmon [6].
Leiomyomas are generally viewed as benign, slow-growing, asymptomatic masses most commonly found in the fourth and fifth decades of life [1]. Signs and symptoms, if present, are related to the site of the tumor and may cause pain [1, 5]. Leiomyomas are more common in women than in men, with a ratio of 3.75:1, a finding that recently has been attributed to presence of estrogen and progesterone receptors in such tumors, leading to the belief that their growth is directly related to female sex hormones [1, 2]. They are most commonly found in female patients of reproductive age with a history of uterine leiomyomas. Benign metastatic leiomyomas also have been described previously, with a minority of reports describing their presence in postmenopausal patients [4, 5, 7].
Head and neck leiomyomas comprise only up to 12% of all leiomyomas due to the paucity of smooth muscles in the head and neck region [1, 2]. Previous studies have described the occurrence of head and neck leiomyomata in the oral cavity, nasal cavity, nasopharynx, larynx, esophagus and orbits [1, 8]. Some reports also have described the presence of leiomyomas in highly unusual regions, such as the epidural space [1, 5]. Several hypotheses exist regarding the origin of such tumors. Primary leiomyomas are believed to arise from aberrant undifferentiated mesenchyme, the tunica media of blood vessels, or both [1, 2].
Surgery is the recommended treatment modality for leiomyomas, with a very low recurrence rate [1, 9]. In the current case, the extraspinal mass encasing the vertebral artery increased the risks associated with further intervention and delayed the second surgery. To the best of our knowledge, this is the second reported case of primary neck leiomyoma not arising from the esophagus. Such tumors should be completely resected from the beginning to avoid progressive growth and secondary complications.



