-
PDF
- Split View
-
Views
-
Cite
Cite
Blaire Anderson, Jordan Nostedt, Safwat Girgis, Tara Dixon, Veena Agrawal, Edward Wiebe, Peter A. Senior, A.M. James Shapiro, Insulinoma or non-insulinoma pancreatogenous hypoglycemia? A diagnostic dilemma, Journal of Surgical Case Reports, Volume 2016, Issue 11, November 2016, rjw188, https://doi.org/10.1093/jscr/rjw188
Close - Share Icon Share
Abstract
Insulinoma is the most common cause of endogenous hyperinsulinemic hypoglycemia in adults. An alternate etiology, non-insulinoma pancreatogenous hypoglycemia (NIPH), is rare. Clinically, NIPH is characterized by postprandial hyperinsulinemic hypoglycemia, negative 72-h fasts, negative preoperative localization studies for insulinoma and positive selective arterial calcium infusion tests. Histologically, diffuse islet hyperplasia with increased number and size of islet cells is present and confirms the diagnosis. Differentiating NIPH from occult insulinoma preoperatively is challenging. Partial pancreatectomy is the procedure of choice; however, recurrence of symptoms, although less debilitating, occurs commonly. Medical management with diazoxide, verapamil and octreotide can be used for persistent symptoms. Ultimately, near-total or total pancreatectomy may be necessary. We report a case of a 67-year-old male with hypoglycemia in whom preoperative workup, including computerized tomography abdomen, suggested insulinoma, but whose final diagnosis on pathology was NIPH instead.
INTRODUCTION
Endogenous hyperinsulinemic hypoglycemia in adults is most commonly secondary to insulinoma islet cell tumors. These tumors are characterized by Whipple's triad of hypoglycemia after fast or exercise, neuroglycopenic symptoms and immediate relief with oral or intravenous glucose administration. A monitored fast with documented hypoglycemia accompanied by symptoms, elevated insulin, C-peptide and proinsulin together confirm the presence of endogenous hyperinsulinemic hypoglycemia [1]. Exogenous hyperinsulinemic hypoglycemia (factitious hypoglycemia), characterized by undetectable proinsulin and C-peptide levels, should be excluded. Urinary or plasma sulfonylureas should also be assessed to rule out surreptitious sulfonylurea ingestion.
Non-insulinoma pancreatogenous hypoglycemia (NIPH) or adult-onset nesidioblastosis, defined as diffuse proliferation of pancreatic islet cells, is a rare cause of hyperinsulinemic hypoglycemia representing only 0.5–5% of such cases [2]. The majority of cases reported in adults occur following bariatric surgery and are thought to be secondary to elevated glucagon-like peptide-1 causing pancreatic islet cell hyperplasia [3–5]. Postprandial hyperinsulinemic hypoglycemia, negative 72-h fasts, negative preoperative localization studies for insulinoma and positive selective arterial calcium infusion tests together are suggestive; however, differentiating insulinoma and NIPH preoperatively remains challenging and ultimately requires histologic assessment.
CASE REPORT
A 67-year-old male retired biochemistry professor presented to the emergency department with a recent history of falls and decreased level of consciousness. He was found to be profoundly hypoglycemic with no antecedent history. Medical history was notable for hypertension, chronic obstructive pulmonary disease, transient complete heart block with bradycardia, alcohol abuse and Wernicke–Korsakoff syndrome. There was no history of surgical intervention, notably no bariatric or gastric procedures. Physical examination was unremarkable.
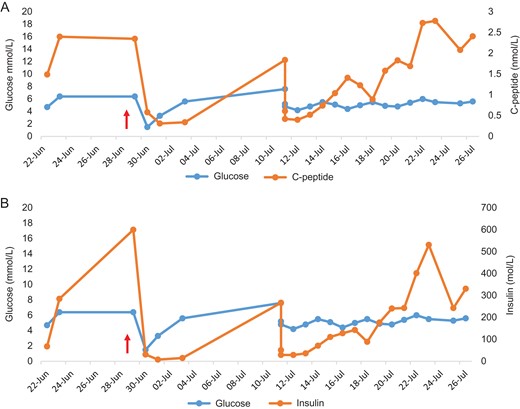
Values obtained during episodes of spontaneous postprandial hypoglycemia initially followed by supervised fasts both preoperatively and postoperatively.
Note, the patient underwent distal pancreatectomy (marked by red arrow). Glucose and C-peptide values (A). C-peptide levels were inappropriately elevated given hypoglycemia. Glucose and insulin values (B). Insulin levels were inappropriately elevated given hypoglycemia.
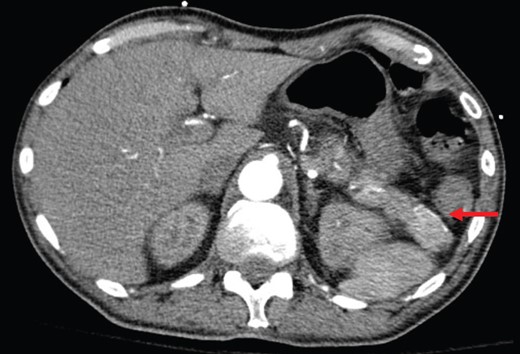
Abdominal CT shows a 2.2-cm arterial enhancing lesion (marked by red arrow) in tail of pancreas consistent with an insulinoma.
Based on sustained hypoglycemia, inappropriate insulin and C-peptide secretion on repeated measures, and the presence of the arterially enhancing mass in the pancreatic tail, a decision was made to proceed with distal pancreatectomy and splenectomy. Intraoperatively, a mass corresponding with preoperative imaging was appreciated. Laparotomy revealed no evidence of other intraabdominal macroscopic disease. Intraoperative ultrasound was considered but not readily available as surgery was carried out in emergency evening hours.
Postoperatively, the patient had persistent hypoglycemia and neuroglycopenia. His pre-existing cognitive issues led to variable oral intake; therefore, ultimately he was stabilized with supplementary nasogastric tube feeds, but ongoing hypoglycemia necessitated diazoxide and acarbose. Additional investigations, including octreotide scan and endoscopic ultrasound of the pancreas, did not show signs of multifocal or metastatic disease.
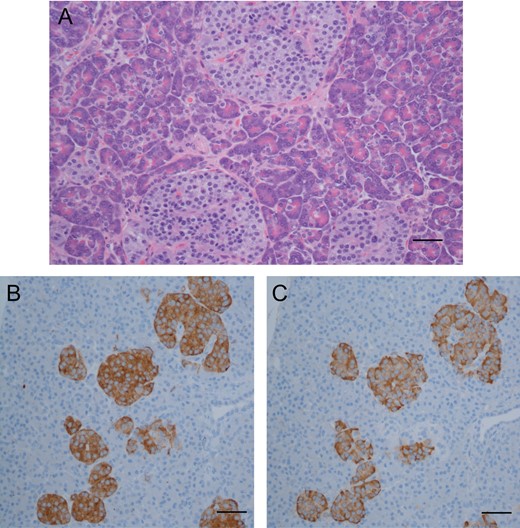
Routine hematoxylin and eosin stain (A) showing subtle increase in number and size of pancreatic islet cells (as viewed at ×400; scale bar indicates 25 μm). Comparison of immunohistochemical stains: synaptophysin (B), staining pancreatic islet cells, and insulin (C), staining beta cells; numerous islet cells consist almost entirely of beta cells (as viewed at ×200; scale bar indicates 100 μm).
DISCUSSION
The differential diagnosis of hypoglycemia is extensive. Appropriate workup will usually elucidate the cause of symptoms. Although rare, one must consider NIPH, particularly following bariatric surgery. NIPH is distinguished from reactive hypoglycemia by development of neuroglycopenic symptoms in addition to the adrenergic symptoms seen in reactive hypoglycemia. NIPH is not associated with the same KATP channel gene mutations as those observed in nesidioblastosis in the pediatric population [6]. However, patch clamp experiments of beta cells from a patient with NIPH demonstrated a state of constant depolarization even at low glucose levels, suggesting a role of KATP channel physiology in the pathogenesis of NIPH [7].
Differentiating NIPH from insulinoma preoperatively remains difficult. Negative imaging studies have been included as a feature of NIPH, although patients may still have microinsulinomas too small to be detected on radiology. Our patient did have a lesion seen on CT, which was palpable at the time of surgery. Gupta et al. [8] describe a similar case where insulinoma was suspected based on clinical picture, preoperative imaging and intraoperative ultrasound but final pathology showed NIPH; by contrast, in their case imaging demonstrated multiple lesions, none of which were grossly detectable intraoperatively.
The majority of patients with NIPH will be controlled by distal pancreatectomy. Early studies suggested a 70% success rate in achieving normoglycemia for NIPH with <10% risk of diabetes mellitus when 60–89% of the pancreas were resected [9]. Gradient-guided distal pancreatectomy following selective arterial calcium stimulation is another technique to aid in extent of resection [10]. Despite amelioration of symptoms and hypoglycemia following pancreatic resection, recurrence has been an issue. A notable study from the Mayo Clinic demonstrated improved quality of life following pancreatic resection; symptom recurrence, when present, was usually less severe and debilitating than previous [3]. Diazoxide can be used when hypoglycemia persists postoperatively. Verapamil and octreotide may be added in cases of persistent hypoglycemia [2]. Ultimately, subtotal or even total pancreatectomy may be pursued if necessary.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
A.M.J.S. is supported through a Canada Research Chair in Transplantation Surgery and Regenerative Medicine from the Canadian Research Council. He also receives funding from Alberta Innovates Healthcare Solutions, a Canadian Clinical Trial Network grant from the Juvenile Diabetes Research Foundation. This work was additionally supported through the Diabetes Research Institute Foundation of Canada (DRIFCan).



