-
PDF
- Split View
-
Views
-
Cite
Cite
Aisling Ní Nualláin, Samuel Ndoro, John Caird, Darach Crimmins, BRAF V600D mutation in a paediatric high-grade glioma, Journal of Surgical Case Reports, Volume 2016, Issue 10, October 2016, rjw150, https://doi.org/10.1093/jscr/rjw150
Close - Share Icon Share
Abstract
The authors report a case of high-grade glioma with unusual pathology that has not previously been reported in glioma pathology. The 5-year-old patient presented to the emergency department with a 1-day history of a right temporal swelling on a background history of increasing nausea and vomiting for the preceding 5 months. A computed tomography brain was performed, which showed a large right-sided temporoparietal lesion. The patient underwent surgery to remove the mass and pathology confirmed anaplastic astrocytoma (WHO Grade 3). This report focuses on prognostic factors in high-grade glioma, particularly on pathological indicators, namely epidermal growth factor receptor, O6-methylguanine-DNA-methyltransferase expression and BRAF V600D mutation.
Introduction
High-grade gliomas, with the majority being of astrocytic lineage, constitute ~20% of all paediatric central nervous system tumours [1]. They most commonly involve the supratentorial hemispheres or the pons. High-grade astrocytomas (HGAs) are much less common in the paediatric population when compared with adults with similar neoplasms. The type of glioma that we will focus on is the anaplastic astrocytoma. Despite an aggressive treatment approach, the 2-year survival rate remains poor: 10–30% for most supratentorial tumours [2].
Case Report
In February 2014, a 5-year old girl presented to the emergency department with a 1-day history of a right temporal swelling, on a background history of vomiting for 5 months. The vomiting was initially associated with motion but progressed to non-motion–related sickness with multiple episodes of vomiting per day, usually subsiding by the afternoon. The patient did not complain of headaches or visual disturbance and did not have any problems with balance or gait. A full neurological examination and examination of all other systems showed no abnormal findings (Figs 1–3).
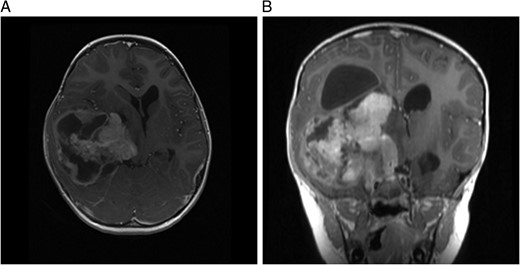
(A) MRI immediately following initial debulking of tumour showing a large subdural collection in a T2 FLAIR image and (B) coronal MRI post-op.
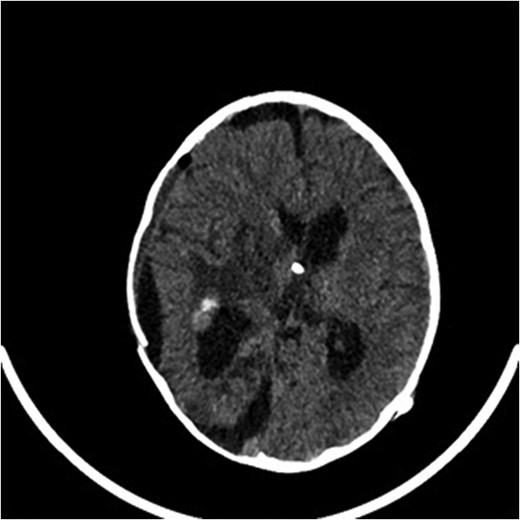
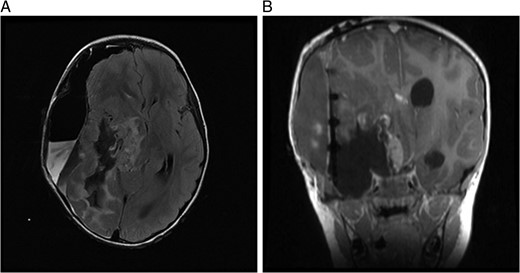
A computed tomography brain showed a large right temporoparietal cystic solid lesion with calcification and mass effect resulting in midline shift. A preoperative magnetic resonance imaging (MRI) brain and spine showed an enhancing lesion that extended to the occipital lobe, measuring 7 cm in maximum diameter (Fig. 1). A cystic lesion, measuring 5 × 8 cm was also identified posterosuperiorly. The medial part of the tumour extended to the thalami and lentiform nucleus. No spinal metastases were found.
A right temporoparietal craniotomy was performed, removing 90% of the tumour with some remaining on the inferior brainstem.
Histology from the frozen section was suggestive of ganglioma. Further analysis revealed a complex glioneuronal tumour consistent with ganglioglioma: anaplastic transformation of glial component (WHO Grade 3). The final diagnosis was a high-grade astrocytic tumour, BRAF fusion not detected, unmethylated with focal amplification of filtration rate epithelial growth factor receptor (EGFR) and a rare two-base-pair substitution affecting V600. This does not code for the usual mutation of V600E but for BRAF V600D which has not previously been reported in a glioma.
MRI 1 month post-op showed that the residual tumour in the basal ganglia, which extended inferiorly to the cerebellar pontine region had grown slightly larger. Also noted was a subdural fluid collection overlying the right cerebral hemisphere with extension into the interhemispheric fissure. Further debulking was carried out on the residual tumour. An MRI immediately post-op showed only 8mm of the tumour lying adjacent to the brainstem remained, which had previously measured 5.2 cm.
Postoperatively, the patient developed right-sided third cranial nerve palsy, left seventh cranial nerve palsy and a left hemiparesis. The deficits are likely attributed to a mild stroke following tumour resection. Further treatment, based on STUPP protocol, began 2 months post-op with a radical radiation therapy regimen and Temozolomide daily during treatment. She received a total dose of 55 Gy in 30 fractions over 6 weeks. She also received adjuvant Temozolomide 200 mg/m2 daily for 5 days in each of the six cycles. Delayed white cell count recovery between cycles prolonged the treatment course. Further surgical management of decompression of the tumour cyst was required in October 2014.
Discussion
The mutation of V600E is reported in ~20% of HGAs. To the best of our knowledge, a V600D mutation has never before been reported in a glioma. It is not known if this will affect the long-term prognosis or if it will respond as well to current therapy strategies. The outcomes for current strategies are largely poor. This is likely related to the fact that there are significant genetic differences in paediatric and adult gliomas. Currently, the majority of treatment is based on adult types of glioma. If the target in paediatric gliomas is not the same as that of which it was originally designed for, it will not have the desired outcome. Attention should be focused on small molecule mutations such as V600E, etc., for new targeted therapies, which may ultimately lead to improved survival rates for paediatric gliomas [1].
EGFR amplification is rare in paediatric gliomas but is the most common genetic abnormality found in adults (~40–50%) [2]. In this case, the patient has histological evidence of EGFR amplification. A study, completed by Smith et al., showed that younger patients (younger than 40 years) with EGFR amplification had shorter survival time than those without. Although this study was not based on paediatrics, the observations suggest that biologic significance of EGFR amplification may be related to patient age and may also be used as a prognostic utility. Further studies would need to be performed in the paediatric population to see if this hypothesis could be extrapolated to this group [3].
A study performed by McDonald et al. has shown that there is a correlation between O6-methylguanine-DNA-methyltransferase (MGMT) expression and outcome in childhood HGAs. The study looked at 10 patients with HGA, 6 of which had unmethylated MGMT promoter. The average survival of these patients was 2.5 months compared to 13.7 months in the patients who had methylated MGMT. There was also noted to be a better response to Temozolomide in patients with methylated MGMT [4].
Degree of surgical resection is the most important factor in determining prognosis. It has been shown to be independent of other prognostic factors, for example histological grade and tumour location. Complete or near-complete (>90%) resection of the tumour typically results in improved overall survival [2]. Of those patients who did not receive full resection, Temozolomide use with concurrent chemoradiation has shown improved survival [5]. In 2004, the European Organisation for Research and Treatment of Cancer (EORTC) conducted a trial that showed overall survival at 2 years was 27.2% with Temozolomide compared to 10.9% with radiotherapy alone. Combined therapy showed a benefit in all clinical prognostic subgroups of the study [6].
It has been 16.5 months since the diagnosis of anaplastic astrocytoma. This patient after having >90% of her tumour resected should have a better prognosis than some. However, this combined with the poorer prognosis of unmethylated MGMT promoter and EGFR amplification may not result in prolonged survival.
Conflict of interest statement
None declared.



