-
PDF
- Split View
-
Views
-
Cite
Cite
Reshma Ghedia, Jonathan A. Dunne, Jessica Steele, Sonja Cerovac, Extensive upper extremity deep venous thrombosis following brief application of an operative arm tourniquet, Journal of Surgical Case Reports, Volume 2016, Issue 10, October 2016, rjw136, https://doi.org/10.1093/jscr/rjw136
Close - Share Icon Share
Abstract
Extensive upper extremity deep venous thrombosis and compartment syndrome secondary to operative tourniquet application are rare outcomes of established practice. We present the case of a 54-year-old female who underwent elective removal of a right olecranon plate under general anaesthetic with brief application of a tourniquet. In recovery, she developed a swollen and erythematous forearm, without significant pain and paraesthesia. An urgent dual-phase computed tomography angiogram identified no venous outflow proximal to the axillary vein. Concern for early compartment syndrome necessitated emergency fasciotomies of the right forearm and hand, precluding thrombolysis. Thrombosis was found in the superficial and deep veins throughout the forearm, but the muscles were healthy. The patient commenced anticoagulation therapy early and made good recovery. Further haematology review concluded that she had a ‘provoked thrombosis’ and no need for long-term anticoagulation.
Introduction
The use of operative tourniquets is integral to upper limb surgery to achieve a bloodless surgical field and complications are rare [1–3]. We present the first case of extensive upper extremity deep venous thrombosis (DVT) and subsequent compartment syndrome secondary to operative tourniquet application. We discuss the aetiology and challenging management of such a case to maintain full function of the hand.
Case Report
A 54-year-old female underwent elective removal of a right olecranon plate due to irritation. Previous upper limb tourniquet application for treatment of a fractured olecranon was uncomplicated. She had a body mass index (BMI) of 27, no other medical conditions, was a non-smoker and had no prior history of coagulopathy. Preoperative blood count and clotting were normal. The procedure was performed under general anaesthetic with a mid-arm tourniquet applied for 34 min at a pressure of 250 mmHg.
After an hour she was noted to have a swollen, erythematous arm to the mid-humeral level. Urgent review by vascular surgery also noted globally reduced sensation but no pain. The arm was warm with a normal capillary refill. Hand-held Doppler confirmed good signal from both radial and ulnar arteries.
Immediate duplex scan found thrombosis of the superficial veins. The deep veins could not be visualized up to the axillary vein, which was patent. Arterial flow was normal. She was given 5000 iU of heparin intravenously 4.5 h following her procedure and urgently transferred to a tertiary vascular and hand surgery service.
Upon further assessment 7 h post-operatively, the forearm was blue and tense with multiple petechiae (Fig. 1). She had diminished sensibility in all fingers but good hand movement. She denied any significant pain. Repeated blood tests confirmed a normal blood count, clotting screen and renal function, but a raised creatine kinase of 1463. An immediate dual-phase computed tomography angiogram confirmed occlusion of the right basilic and axillary veins with poor compensatory flow through the cephalic vein. The arterial phase was normal.
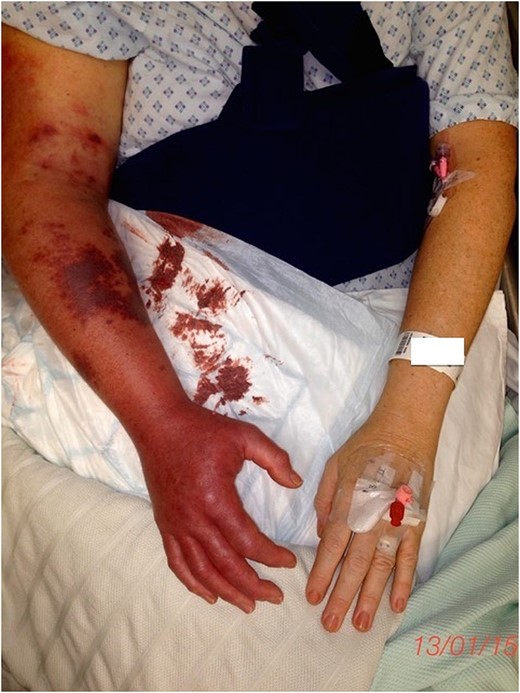
The concern of early compartment syndrome necessitated emergency fasciotomies, which were performed 8 h post-operatively (Fig. 2). Compartment pressure monitoring was unavailable. Muscles were healthy despite extensive thrombosis found within the superficial and deep veins throughout the arm. Therefore, attempted intraoperative embolectomy was unsuccessful. The patient continued an intravenous infusion of unfractionated heparin post-operatively.
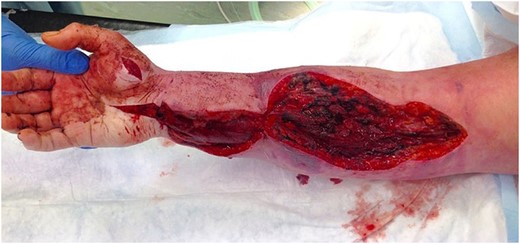
Venous outflow improved and a second assessment in theatre was performed at 36 h. Skin and muscle were healthy and topical negative pressure dressing was applied over the fasciotomy incisions. Swelling continued to improve and on Day 7 a split-thickness skin graft was used to close the fasciotomies (Fig. 3). The heparin was converted to rivaroxaban after 7 days. She was discharged after 3 weeks but continued oral anticoagulation and hand physiotherapy in the community. When seen in clinic she had normal range of movement and function of the upper limb.
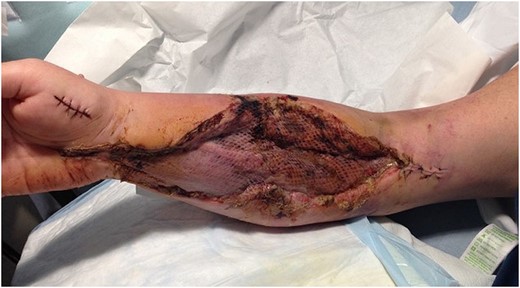
Split-thickness skin graft used to close the fasciotomy wounds.
Outpatient haematology review at 3 months concluded that the event was a ‘provoked thrombosis’ and further investigation was unnecessary.
Discussion
Upper limb DVT can be divided into two categories: primary and secondary. About 66–80% are thought to be secondary with risk factors including central venous catheters, surgery or trauma and hypercoagulable states, such as malignancy and inherited disorders [4, 5].
The use of tourniquets is integral to upper limb surgery to achieve a bloodless surgical field. Complications of their use secondary to trauma and biochemical changes include swelling, weakness, compression neuropraxia, vascular injury, tissue necrosis and compartment syndrome but are rare [1]. Safe duration and pressures for tourniquet use were first reported by Brunner in 1951 and modified most recently in 2002 by Kutty and McElwain [6]. They advise tourniquet application at pressures of 200–250 mmHg on the arm for no longer than 2 h. In our case, the patient had the tourniquet applied for 34 min at 250 mmHg, well within recommendations. Studies have indeed shown no statistical significance in the incidence of DVT with tourniquet use [2, 3]. There has only been one documented case of an extensive upper limb venous thrombosis with tourniquet use, although this was in the context of prior trauma with an associated haematoma [7]. We describe the first case of extensive upper limb DVT following elective tourniquet use in a patient with no significant risk factors for coagulopathy.
Our management priorities for the patient focused on prevention of muscle necrosis, further thrombosis and embolism and post-thrombotic syndrome. Current recommendations in the literature recommend early anticoagulation with heparin or fondaparinux for a proximal upper extremity deep vein thrombosis with subsequent thrombolysis or thrombectomy if symptoms warrant plus removal of precipitants [8, 9]. In this case, anticoagulation was commenced 3.5 h after the onset of symptoms. Clinical assessment suggested an early compartment syndrome and so emergency fasciotomies were the priority to prevent muscle necrosis. This contraindicated medical thrombolysis that would have lead to profound blood loss and so intraoperative embolectomy was attempted. This was unsuccessful due to the extent of thrombosis. Three months of anticoagulation were ultimately completed with heparin and then rivaroxaban. This illustrates that despite failed thrombectomy and contraindication of thrombolysis, early anticoagulation was successful to re-establish venous outflow and achieve good functional outcome.
Haematology review at 3 months concluded it was a single ‘provoked thrombosis’ for which a 3-month course of rivaroxaban was sufficient and further investigation, including for Factor V Leiden was unwarranted. She was however still advised to avoid oral-contraceptive medication. The pathophysiology of thrombus formation is considered 3-fold that together multiply the thrombotic risk. Firstly, surgery in itself provokes a hypercoagulopathic state, which increases thrombotic risk [2, 10]. Not only does surgery lead to stasis but there is also physical disruption of endothelium, which exposes extravascular tissue factor, thereby activating the intrinsic coagulation cascade. Additionally, the stress of surgery releases inflammatory mediators such as cytokines, chemokines, vascular endothelial growth factors and complement factors, which activate the extrinsic pathway [2]. Secondly, tourniquets exacerbate both stasis and release of inflammatory mediators by compression. This activates leucocytes and releases thrombotic markers such as plasmin, D-dimer, tissue plasminogen activator, angiotensin-converting enzyme, antithrombin-III and protein C [1]. Thirdly, the patient had a BMI of 27 and it has been repeatedly been shown that overweight patients are at increased risk of DVT, especially when this risk is combined with others factors, such as surgery [10].



