-
PDF
- Split View
-
Views
-
Cite
Cite
Dalal Al-Faraj, Mohanned Al-Haddad, Omar Al-Hadeedi, Saud Al-Subaie, A case of acute mesentero-axial gastric volvulus in a patient with a diaphragmatic hernia: experience with a laparoscopic approach, Journal of Surgical Case Reports, Volume 2015, Issue 9, September 2015, rjv119, https://doi.org/10.1093/jscr/rjv119
Close - Share Icon Share
Abstract
Gastric volvulus is an uncommon but serious surgical condition mandating an early diagnosis and surgical intervention. It may present either acutely or chronically with epigastric pain, retching and vomiting. There are two types of gastric volvulus: organo-axial and mesentero-axial. We report a case of a mesentero-axial gastric volvulus in a 49-year-old woman with a left-sided diaphragmatic hernia. She presented with a significant epigastric pain and vomiting. A flexible upper endoscopy, a barium meal and a contrast-enhanced computed tomography imaging had confirmed the diagnosis. She was treated with a laparoscopic mesh repair of the diaphragmatic defect followed by a gastropexy. She had an uneventful postoperative course and was asymptomatic thereafter.
INTRODUCTION
Gastric volvulus is characterized by a rotation of the stomach along its axis in variable degrees and may present either acutely or chronically. It may cause a complete gastric outlet obstruction and strangulation, leading to ischemic necrosis and perforation with a mortality rate of 30–50% [1].
Primary gastric volvulus results from abnormalities of the four gastric ligaments that restrict rotation: gastrocolic, gastrophrenic, gastrosplenic and hepatogastric [2]. Secondary gastric volvulus results from anatomical anomalies including para-oesophageal and diaphragmatic hernias [3].
We report a case of a gastric volvulus in a patient with a history of a diaphragmatic hernia. An emergent laparoscopic diaphragmatic hernia mesh repair and gastropexy was performed, resulting in a complete recovery.
This case is presented to support the feasibility of the laparoscopic approach.
CASE REPORT
A 49-year-old woman presented to our emergency department with a 2-week history of epigastric pain and vomiting with no prior trauma. Her past medical history included hypertension, iron deficiency anemia, laparoscopic cholecystectomy and an open uterine myomectomy. Upon arrival to the emergency department, she had a pulse of 135 beats per minute, a blood pressure of 110/90 mmHg, a respiratory rate of 20/min, a body temperature of 37.6°C and an oxygen saturation of 98% on room air. Her clinical examination revealed tenderness over the epigastric region, and her bowel sounds were hyperactive. She had decreased air entry at the left basal region of the chest. The initial laboratory results were white blood cell count 22.1 × 109/l (neutrophilic), blood urea nitrogen 7.8 mmol/l, creatinine 121 umol/l and normal liver function tests, amylase and lipase. Her lactic acid was 0.9 mmol/l. A chest radiograph demonstrated a significant air-fluid level near the left lung base (Fig. 1).
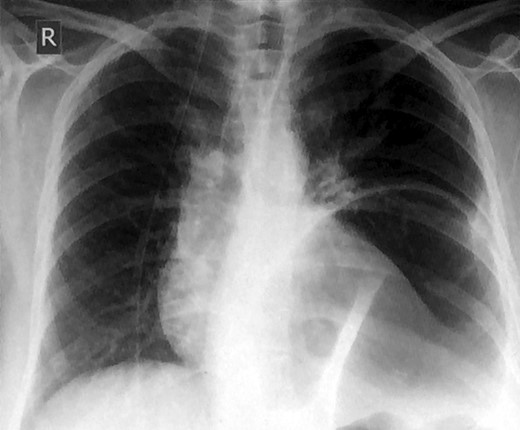
Chest x-ray showing an elevated air-fluid left in the left basal lung region.
Two days prior to her admission, she had undergone upper gastrointestinal endoscopy for the same complaint in another center that revealed a dilated gastric lumen with herniation of the distal stomach, pylorus and first part of the duodenum into the left hemi-thorax (Fig. 2). She refused treatment and left against medical advice. She presented to our emergency department with worsening symptoms. Resuscitation was commenced with immediate nasogastric decompression, intravenous hydration and analgesia, proton-pump inhibitors and electrolyte replacement. A double contrast-enhanced computed tomography (CT) scan of the chest and the abdomen demonstrated a large diaphragmatic defect (7 × 7 cm) and a left diaphragmatic hernia containing a dilated stomach, suggestive of a gastric outlet obstruction (Fig. 3). A water-soluble gastrografin meal was performed, showing an intra-thoracic, mesentero-axial gastric volvulus (Fig. 4).
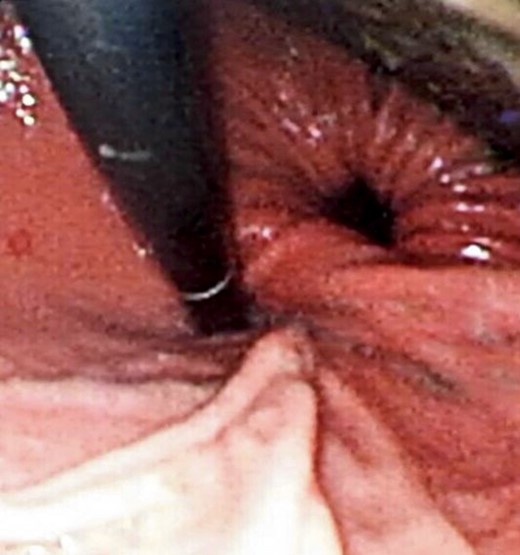
Flexible upper endoscopy showing herniation of the distal stomach, the pylorus and the first part of the duodenum into the left hemi-thorax.
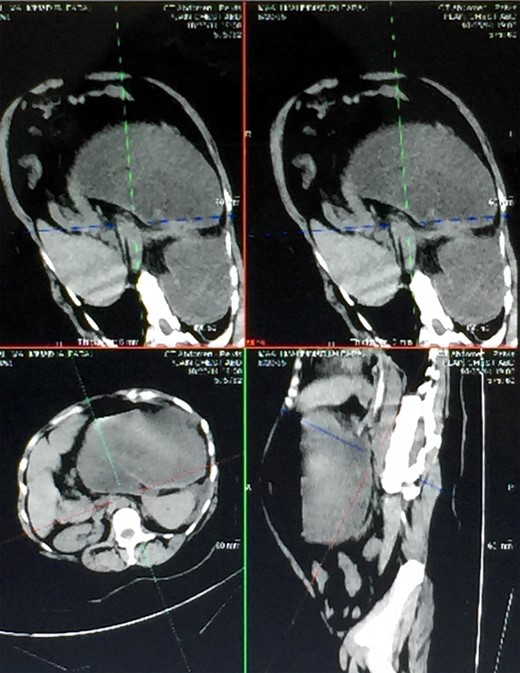
Different views of a double contrast-enhanced CT scan showing a left diaphragmatic hernia with an acute dilated stomach.
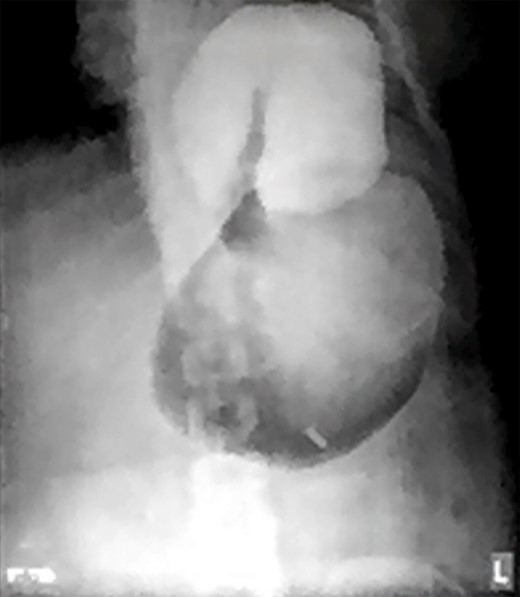
Water-soluble gastrografin meal showing an intra-thoracic mesentero-axial volvulus.
The patient was listed emergently, underwent general anesthesia and was placed in a 30-degree split-leg reverse Trendelenburg position. A four-port approach was used after Verres pneumoperitoneum was achieved. On inspection of the peritoneal cavity, the stomach and the first part of the duodenum were incarcerated in the peritoneal sac in the left hemi-diaphragm (Fig. 5). Adhesiolysis was performed around the defect using a harmonic scalpel. The rotated stomach and the first part of the duodenum were completely reduced from the thoracic cavity into the abdomen. Once the dissection of the sac was complete, we confirmed that there was no visceral injury and proceeded to repair of the 7 × 7 cm diaphragmatic hernia (Fig. 6). The defect was closed primarily using an interrupted polydioxanone suture (Fig. 7). A GORE® BIO-A® Tissue Reinforcement absorbable mesh (15 × 12 cm) was selected to encourage the formation of vascularized neotissue. Furthermore, this mesh can be used safely in contaminated fields [4]. It was fixed in place using ETHICON SECURESTRAP® Absorbable Strap Fixation Device (Fig. 8). A gastropexy was then performed to the anterior abdominal wall using a 2-0 polypropylene suture.
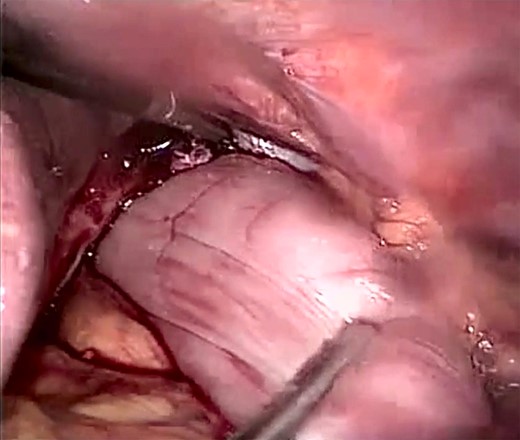
Laparoscopic view showing the incarcerated content in the peritoneal sac with a twisted stomach.
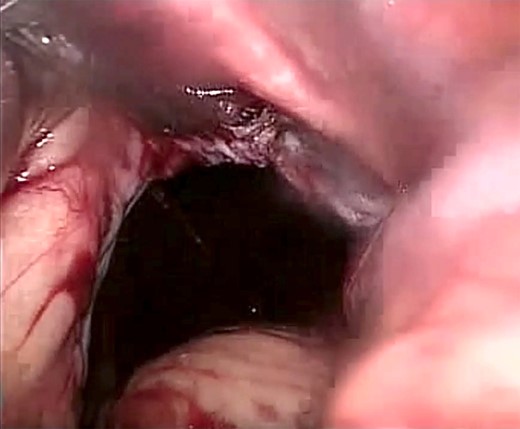
Laparoscopic view showing a large (size 7 × 7 cm) diaphragmatic defect.
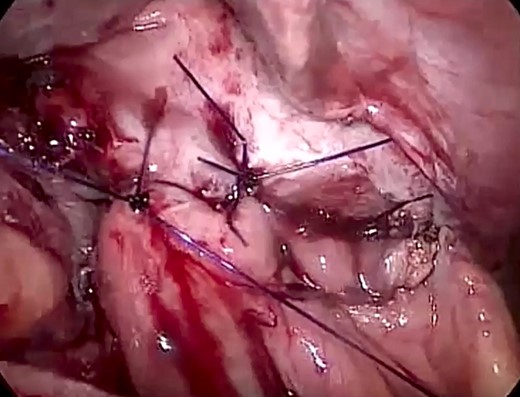
Laparoscopic view showing the primary closure of the diaphragmatic defect using an interrupted PDS™ II Suture.
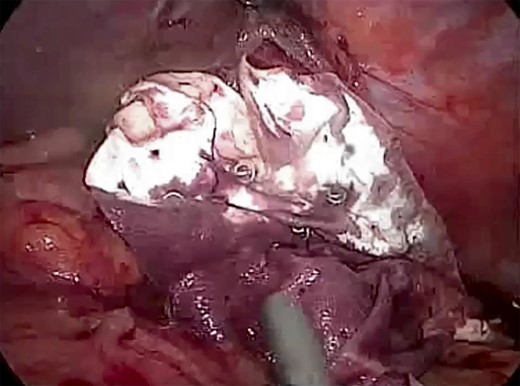
Laparoscopic view showing BIO-A® Tissue Reinforcement absorbable mesh (size 15 × 12 cm) fixed intraperitoneally below the defect.
On the first post-operative day, a water-soluble meal study was repeated and revealed a marked regression of the stomach size and a normal intra-abdominal position of the stomach and the first part of the duodenum with no evidence of obstruction (Fig. 9). The patient had an unremarkable recovery and was discharged on post-operative day 3. She was followed up in clinic 2 weeks post-operatively, and a follow-up upper gastrointestinal endoscopy was performed, which showed normal anatomy to the second part of the duodenum (Fig. 10). The patient returned to her activities of daily living.
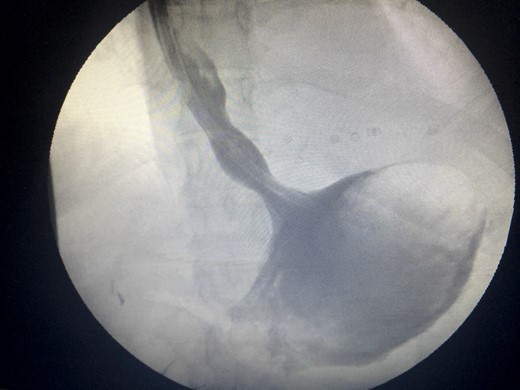
Water-soluble gastrografin meal showing normal intra-abdominal position of the stomach.
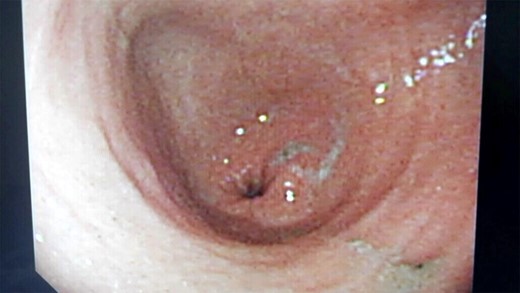
Follow-up flexible upper endoscopy showing normal gastric anatomy.
DISCUSSION
The majority of patients with gastric volvulus seek care when symptoms occur. A detailed history of the risk factors associated with gastric volvulus including previous surgical procedures, gastro-oesophageal reflux disease or history of trauma should be obtained. A plain chest radiograph usually shows a single, elevated air-fluid level. Other more sensitive imaging modalities are CT scan and barium meal studies [5]. The initial management includes nasogastric decompression (endoscopically if necessary), fluid resuscitation and correction of electrolyte imbalances [1, 6].
Endoscopic decompression, de-rotation and percutaneous endoscopic gastrostomy (PEG) fixation of the stomach to the posterior abdominal wall are the first-line management for cases of primary gastric volvulus. It is also employed in cases of secondary gastric volvulus who are unfit for surgical repair under anesthesia. The use of two PEG tubes is optimal as the stomach may still rotate over a single PEG [7].
Surgical intervention is the treatment of secondary gastric volvulus and cases of volvulus in which an endoscopic or nasogastric decompression and de-rotation have failed. Open surgical repair was the historical treatment of choice. There are no randomized control trials available; however, observational studies support laparoscopy for its benefits of reduced inpatient stays and improved post-operative morbidity [8]. A recurrence rate of 42 vs. 15% post laparoscopic and open gastropexy, respectively, has been described in one study [8]. However, it is important to stress that this is for gastric fixation alone and does not compare recurrence rates after hernia repair [9].
The laparoscopic approach is defined by the need to de-rotate and reduce the gastric contents into the abdominal cavity. Repair of anatomical defects is then accomplished. Smaller defects may be closed by primary repair with intra-corporeal sutures. Larger defects not permitting closure without tension should be repaired with a mesh. Fixation by suture gastropexy or laparoscopically placed PEG tube reduces recurrence rates. A partial gastrectomy may be required to resect necrosis [6].
The role of laparoscopy is evolving as a technique in this uncommon surgical emergency.
CONFLICT OF INTEREST STATEMENT
None declared.



