-
PDF
- Split View
-
Views
-
Cite
Cite
Fuyuki F. Inagaki, Yoshiaki Hara, Masako Kamei, Michio Tanaka, Masamichi Yasuno, Acute and chronic acalculous cholecystitis associated with aortic dissection, Journal of Surgical Case Reports, Volume 2015, Issue 8, August 2015, rjv101, https://doi.org/10.1093/jscr/rjv101
Close - Share Icon Share
Abstract
Acalculous cholecystitis is a rare but life-threatening disease, but its pathogenesis is not fully revealed yet. We experienced two acalculous cholecystitis cases associated with aortic dissection. In Case 1, acalculous cholecystitis occurred just after the exacerbation of the aortic dissection. Laparotomy showed necrotized cholecystitis with fresh thrombi formation. Case 2 developed acalculous cholecystitis on the 65th hospital day of aortic dissection. Laparotomy revealed the perforation of the gallbladder. Histological study revealed fibrosis and hemosiderosis in the subserosal layer. The histological findings of these two patients are quite different: Case 1 is acute ischemic and Case 2 is chronic ischemic. While a few cases of acute ischemic cholecystitis have been reported previously, chronic acalculous cholecystitis (CAC) has not been documented. History of aortic dissection could be a risk factor of acute and CAC due to relatively decreased splanchnic blood flow.
INTRODUCTION
Acute acalculous cholecystitis (AAC) is defined as inflammation of the gallbladder in the absence of gallstones, which constitutes 2–15% of all cases of acute cholecystitis [1, 2]. It often occurs in patients with multiple trauma, burns, recent operations for non-biliary tract disease, sepsis, shock of any kind, total parenteral nutrition and prolonged fasting. Moreover, according to the recent reports, the number of AAC patients has been increasing among outpatients. Compared with acute calculous cholecystitis, AAC often runs a fulminating course because of the high incidence of necrosis and perforation. Therefore, delays in diagnosis and treatment lead to a high mortality rate. We recently diagnosed two cases of acalculous cholecystitis, which were caused by aortic dissection.
CASE REPORT
Case 1
A 69-year-old male who had an 8-year history of Stanford type B aortic dissection presented with vomiting and abdominal pain. Physical examination revealed right upper abdominal tenderness, but no muscular defense. Blood tests revealed leukocytosis and elevated levels of amylase, but other laboratory data were within normal range. Abdominal sonography showed the inflammatory thickened gallbladder wall without any gallstones and biliary sludge. Contrast-enhanced computed tomography (CT) also showed the enlargement of the false lumen compared with a study 5 years earlier. The gallbladder and pancreatic body and tail were swollen, indicating acute pancreatitis and AAC (Fig. 1A). A highly enhancing lesion was detected in the gallbladder, which appeared to be the gallbladder hemorrhage. Laparotomy revealed necrotized cholecystitis with dominant histopathologic changes at the fundus of the gallbladder (Fig. 1B). Pathological exams showed partial mucosal defect and submucosal bleeding in the whole gallbladder wall (Fig. 1C). In addition, fresh thrombi formation was found in the arterioles at the fundus and less frequently in the body/neck of the gallbladder (Fig. 1D).
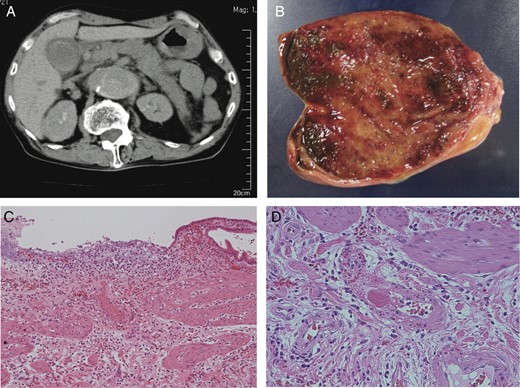
(A) CT showed Stanford type B aortic dissection, gallbladder wall thickness without gallstones and swollen pancreas. (B) Macroscopic examination showed the necrosis of the gallbladder fundus. (C) Histological examination revealed that partial mucosal defect, mucosal/submucosal arterial thrombi and submucosal bleeding in the body of the gallbladder wall (H&E ×100). (D) Histological examination revealed the fresh thrombi formation in the arterioles at the fundus of the gallbladder (H&E ×400).
Case 2
A 74-year-old male who complained of acute back pain was admitted to the hospital. He was diagnosed with Stanford type B aortic dissection and immediately underwent conservative treatment. On 65th hospital day, he suddenly developed high fever and severe right hypochondrial pain with local muscular defense, but laboratory data were within normal range. In the sonographic study, the gallbladder wall was not thickened, and neither gallstones nor biliary sludge was detected. However, contrast-enhanced CT demonstrated acalculous cholecystitis with the partial defect of the gallbladder fundus wall. Small fluid collection was found around the deficit (Fig. 2A). Laparotomy showed the perforation of the gallbladder fundus (Fig. 2B). Although no gallstone was found in the resected specimen, histopathological study revealed fibrosis and hemosiderosis in the subserosal layer (Fig. 2C and D). Intimal fibrotic change of arterioles was dominant at the peripheral part of the gallbladder. There was no pathological finding of vasculitis.
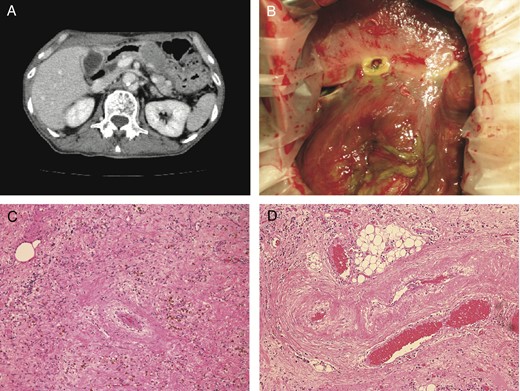
(A) CT showed Stanford type B aortic dissection, the wall defect of gallbladder fundus and the local fluid collection. (B) Laparotomy showed the perforation of the gallbladder fundus. (C) Histological examination showed fibrosis and hemosiderosis in the subserosal layer (H&E ×100). (D) Fibrotic change of arterioles was dominant at the peripheral part of the gallbladder (H&E ×100).
DISCUSSION
Although the pathogenesis of AAC has not been sufficiently clarified yet, several factors have been suggested as possible mechanisms of this disease [3]. Not only bile stasis due to increased bile viscosity but also ampullary constriction due to narcotics or edema of the ampulla of Vater can lead to the increase in intraluminal pressure of the gallbladder. Positive pressure ventilation might increase pressure within the common bile duct in an experimental study [4]. In addition, some reports suspected that ischemia played a central role in the pathogenesis of AAC [5]. The gallbladder possesses a terminal artery, so the decrease in gallbladder perfusion pressure by visceral hypoperfusion might easily lead to ischemia of the gallbladder mucosa. Hakala et al. demonstrated that capillary flow was regular in gallstone-related cholecystitis, but in AAC the capillary flow was poor and irregular [6]. Thus, they claimed that AAC should be called as ‘acute ischemic cholecystitis’.
To our knowledge, only two cases of AAC clearly caused by aortic dissection have been reported previously [7, 8]. Pertinent details of these cases including our two cases are summarized in Table 1. The pathology of these previous cases is similar to our Case 1. Roth et al. reported that microscopic examination revealed deep acellular necrosis of the gallbladder wall with fresh thrombi in the small vessels, accompanied by slight polymorphonuclear infiltration. Sög˘ütlü et al. reported that the gallbladder was completely necrotic with acellular transmural severe necrosis. Case 1 showed fresh thrombi formation within the arterioles and partial mucosal defect in the gallbladder wall. These histological findings are characteristic for acute ischemic change. Roth et al. reported that aortic dissection could induce thrombosis of the cystic artery on the basis of low splanchnic flow. We speculate a similar cause for the Case 1 because the patient had acute pancreatitis mainly in the body and tail of the pancreas simultaneously.
Summary of the four reported cases of acalculous cholecystitis associated with aortic dissection
| . | Age/sex . | Aortic dissection type . | Onset . | Treatment . | Pathology . |
|---|---|---|---|---|---|
| Roth et al. | 57M | de Bakey type III | Soon after | Open cholecystectomy | Deep acellular necrosis with fresh thrombi in the small vessels, slight polymorphonuclear infiltration, gangrenous without perforation |
| Gokhan et al. | 62M | de Bakey type III | Same day | Open cholecystectomy | Completely necrotic |
| Case 1 | 69M | Stanford type B | Same day | Open cholecystectomy | Partial mucosal defect, submucosal bleeding, fresh thrombi in the arteriole |
| Case 2 | 74M | Stanford type B | 2 months later | Open cholecystectomy | Perforation of fundus, fibrosis and hemosiderosis in the subserosal layer |
| . | Age/sex . | Aortic dissection type . | Onset . | Treatment . | Pathology . |
|---|---|---|---|---|---|
| Roth et al. | 57M | de Bakey type III | Soon after | Open cholecystectomy | Deep acellular necrosis with fresh thrombi in the small vessels, slight polymorphonuclear infiltration, gangrenous without perforation |
| Gokhan et al. | 62M | de Bakey type III | Same day | Open cholecystectomy | Completely necrotic |
| Case 1 | 69M | Stanford type B | Same day | Open cholecystectomy | Partial mucosal defect, submucosal bleeding, fresh thrombi in the arteriole |
| Case 2 | 74M | Stanford type B | 2 months later | Open cholecystectomy | Perforation of fundus, fibrosis and hemosiderosis in the subserosal layer |
Summary of the four reported cases of acalculous cholecystitis associated with aortic dissection
| . | Age/sex . | Aortic dissection type . | Onset . | Treatment . | Pathology . |
|---|---|---|---|---|---|
| Roth et al. | 57M | de Bakey type III | Soon after | Open cholecystectomy | Deep acellular necrosis with fresh thrombi in the small vessels, slight polymorphonuclear infiltration, gangrenous without perforation |
| Gokhan et al. | 62M | de Bakey type III | Same day | Open cholecystectomy | Completely necrotic |
| Case 1 | 69M | Stanford type B | Same day | Open cholecystectomy | Partial mucosal defect, submucosal bleeding, fresh thrombi in the arteriole |
| Case 2 | 74M | Stanford type B | 2 months later | Open cholecystectomy | Perforation of fundus, fibrosis and hemosiderosis in the subserosal layer |
| . | Age/sex . | Aortic dissection type . | Onset . | Treatment . | Pathology . |
|---|---|---|---|---|---|
| Roth et al. | 57M | de Bakey type III | Soon after | Open cholecystectomy | Deep acellular necrosis with fresh thrombi in the small vessels, slight polymorphonuclear infiltration, gangrenous without perforation |
| Gokhan et al. | 62M | de Bakey type III | Same day | Open cholecystectomy | Completely necrotic |
| Case 1 | 69M | Stanford type B | Same day | Open cholecystectomy | Partial mucosal defect, submucosal bleeding, fresh thrombi in the arteriole |
| Case 2 | 74M | Stanford type B | 2 months later | Open cholecystectomy | Perforation of fundus, fibrosis and hemosiderosis in the subserosal layer |
Histological study of AAC showed that neutrophils margination of blood vessels and lymphatic vessel dilatation were observed, indicating the existence of ischemic and reperfusion-mediated injury [9]. On the other hand, Case 2 presented with perforation of the fundus of the gallbladder with relatively slight acute inflammation and infiltration of lymphocytes. Long-periods chronic ischemia leads to mucosal atrophy and wide range of fibrosis of lamina propria or deeper, including partial hyalinization. Histological study of Case 2 also showed fibrosis and hemosiderosis in the subserosal layer and chronic fibrotic change of arterioles, which was compatible with histological change of chronic ischemia. That is to say, Case 2 would be more appropriately called ‘chronic acalculous cholecystitis (CAC)’. Recent reports showed that the number of acalculous cholecystitis in outpatients was increasing, and the outcome of these patients was much better than that of acalculous cholecystitis in hospital inpatients [10]. The difference of the outcome was unknown, but might be explained by the difference of the disease entity; acute and chronic. Further histological study would be needed.
The decrease in splanchnic blood flow due to aortic dissection could cause the acute and chronic ischemia in the peripheral part of the gallbladder. Therefore, the history of aortic dissection could be a risk factor of both AAC and CAC. Immediate surgical approach is desirable to avoid poor prognosis.
CONFLICT OF INTEREST STATEMENT
None declared.



