-
PDF
- Split View
-
Views
-
Cite
Cite
Sandrine Dackam, Katarzyna Furrer, Martin Haug, D. Lardinois, Diffuse lymphatic leakage after continuous vacuum-assisted closure therapy for thoracic wound infection after rib stabilization, Journal of Surgical Case Reports, Volume 2015, Issue 12, December 2015, rjv155, https://doi.org/10.1093/jscr/rjv155
Close - Share Icon Share
Abstract
Vacuum-assisted closure (VAC) therapy is a useful tool in the management of a wide spectrum of complex wounds in cardiothoracic surgery. It promotes healing through the application of a controlled and localized negative pressure on porous polyurethane absorbent foams. Known advantages of the VAC therapy are the acceleration of wound healing, stimulation of granulation tissue and reduced tissue edema. Despite its excellent properties, some related complications after and during the therapy have been reported. We report the case of a 47-year-old female with a thoracic wound infection after rib stabilization, managed with open surgery and VAC therapy, which was complicated by a diffuse lymphatic leakage. This is the first case described of diffuse lymphatic leakage followed by partial necrosis of the breast after continuous VAC therapy. We recommend the application of a lower pressure level of this device for complex wounds of the chest wall near the breast.
INTRODUCTION
Wound healing is a complex and dynamic process that includes an immediate sequence of cell migration leading to repair and closure. Vacuum-assisted closure (VAC, KCI International, San Antonio, TX, USA) therapy was first reported in 1997. It has become a common modality for treating complex wounds, characterized by a controlled and localized negative pressure applied on polyurethane absorbent foams [1]. Open-pore polyurethane foams consist of 400–600-µm-sized pores. These pore sizes are the most effective at transmitting mechanical forces across the wound and provide an even distribution of negative pressure over the entire wound bed to aid in wound healing. VAC therapy has shown promising results in several studies, in terms of the wound-healing process and for the treatment of infection after cardiothoracic surgery [2]. However, this method can have some disadvantages like pain, bleeding, heart rupture, death, deterioration in quality of life, loss of protein, fistula formation and secondary infection. Lymphatic complications such as lymphocele and lymphatic leakage are common after vascular interventions and surgeries, and VAC therapy is used as the primary method of treatment. In this report, we present a case of a patient with thoracic wound infection after rib stabilization that was complicated by a diffuse lymphatic leakage and lymphedema of the breast after continuous VAC therapy. This very rare complication of the VAC therapy did not promote healing, but further hindered it.
CASE REPORT
A 47-year-old female visited our clinic with dislocated rib serial fractures with flail chest and hematothorax (Fig. 1A) on the left side, following severe blunt trauma. The medical history was positive for obesity (body mass index 32.2 kg/m2). Stabilization of four rib fractures (Fig. 1B) was performed with StraTos™ (MedXpert GmbH, Heitersheim, Germany) with 3D rib clips and connecting bars for overbridging fragment fixation via an anterolateral approach after diagnostic thoracoscopy and hematothorax evacuation. Immediate postoperative recovery was uneventful. Ten days after the operation, the patient developed a wound infection. A broad-spectrum antibiotic therapy was administered, and a surgical revision with large debridement was performed. The osteosynthetic material was not removed. To close the infra-mammary wound temporarily and to accelerate wound healing, VAC therapy was initiated (Fig. 1C). The site was sealed with an adhesive drape covering the polyurethane foam and tubing, including 4–5 cm of surrounding healthy tissue to ensure a seal. A controlled continuous pressure of 75–125 mmHg was uniformly applied to all tissue on the inner surface of the wound. This system was subsequently renewed every 3–5 days under general anesthesia for a total of 6 times. The wound was reopened, and the VAC foam was removed and discarded. A microbiology culture swab was taken before wound irrigation with normal saline. Necrotic tissue was removed during surgical debridement that was performed when necessary, and adequate hemostasis was achieved. Over the course, the patient developed a diffuse lymphatic leakage and congestion of the left breast with partial necrosis (Fig. 2A) of the lower breast quadrants, requiring repeated surgical debridement and necrosectomy (Fig. 2B).
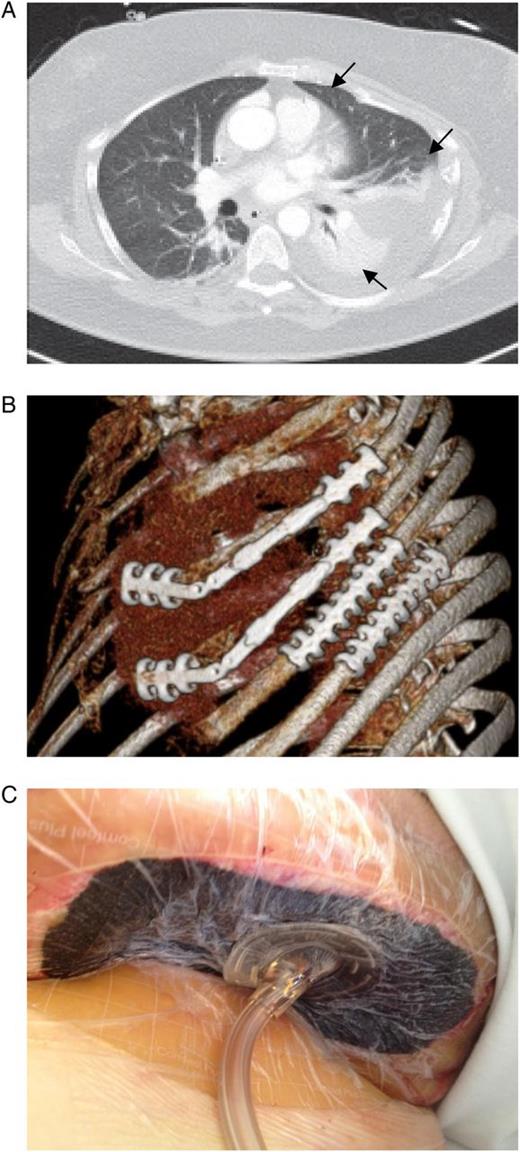
(A) Computed tomography scan of the chest with multiple displaced anterolateral rib fractures and hematothorax (arrow). (B) Osteosynthesis of four ribs with StraTos™ (MedXpert GmbH, Heitersheim, Germany). (C) Applied VAC® system therapy.
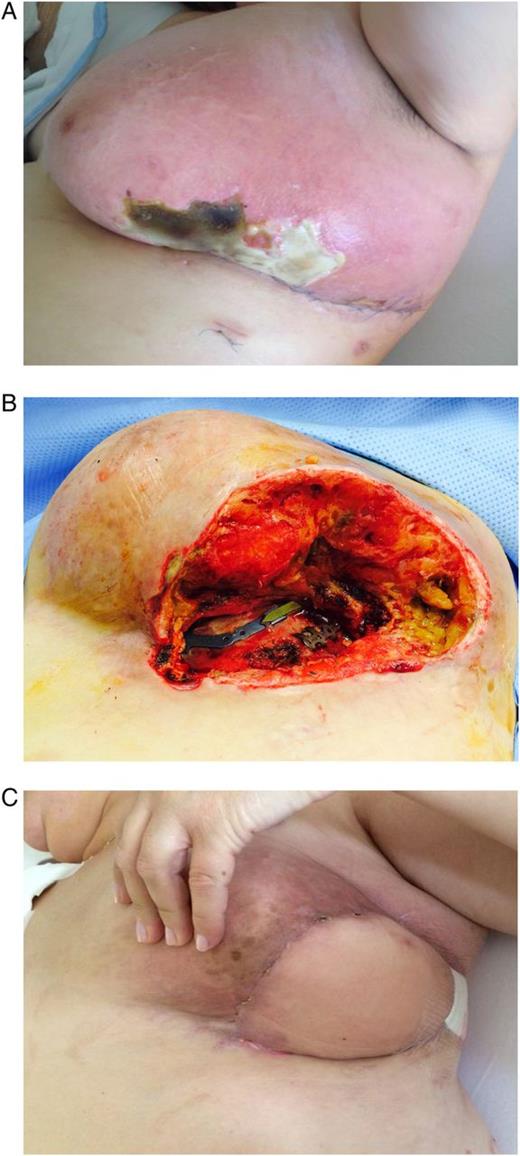
(A) Wound infection with partial necrosis and lymphatic leakage with lymphedema of the left breast. (B) Status after repeated surgical wound debridement and necrosectomy. (C) Result after breast reconstruction with pedicled myocutaneous Latissimus dorsi island flap.
For reconstruction of the skin and soft tissue defects of the breast, a pedicled myocutaneous Latissimus dorsi island flap was performed (Fig. 2C). At 6 months, no other treatments were necessary, the patient was asymptomatic and the follow-up was uneventful.
DISCUSSION
VAC therapy of complex acute- and chronic-infected wounds has increased in popularity among various surgical specialties. There are few reports of VAC therapy for treating open thoracic wounds after rib stabilization [3], and we experienced an interesting case of lymphatic leakage after introduction of the VAC therapy. VAC therapy was applied in our patient on Day 10 after rib stabilization during wound revision due to a local infection of the infra-mammary wound. In general, the adverse events of VAC therapy are seldom but can be life-threatening. These include fistula formation, bleeding as well as secondary infection and death [4].
Our case illustrates a first-described complication of exacerbated diffuse lymphatic leakage with lymphedema and necrosis of the breast in relation to the VAC therapy, and the following reconstructive treatment resulted in prolongation of the hospital stay. We suggest that this complication occurred due to a too high negative pressure application of the VAC system. The level of applied pressure causes a change in microvascular blood flow, and the high negative pressure can result in an impairment of the lymphatic drainage of the breast. This causes lymphedema with congestion of protein-rich fluid, an increase in the size and the number of lymphatic channels and a reduction in the availability of oxygen. This milieu provides a rich medium for bacterial growth resulting in infection, e.g. cellulitis, lymphangitis, lymphadenitis and necrosis of the breast with secondary damage of the lymphatic system. VAC has been used successfully for difficult breast wounds such as infections after mammoplasty [5], before reconstruction of the breast after removal of a giant cystosarcoma phyllodes [6], necrotizing fasciitis of the breast [7], thoracic wall recurrence after breast cancer [8] and mastitis [9]. Stoeckel et al. [10] presented 18 cases of complicated breast wounds requiring VAC. In 2 of these 18 cases, VAC therapy was unsuccessful as the wounds were further complicated by tissue ischemia and infection requiring operative debridement. Unfortunately, Stoeckel et al. [10] do not give further information regarding these two cases that appear to have a similar etiology as the study subject in our case report. The wound vasculature of these two patients was significantly compromised as is the case with our study subject. Since the VAC pressure used is not mentioned, it is difficult to compare the various studies [6, 8–10].
We recommend using VAC therapy at a lower negative pressure of, for example, 50 mmHg, to avoid this rare complication. Future research should focus on improvement of the device to avoid complications and expand clinical indications. Furthermore, high-quality randomized clinical trials with standardized controls and protocols are needed to compare outcomes in specific types of patients and wounds, at different levels of pressure, and using different pressure models adapted to wound composition.
CONFLICT OF INTEREST STATEMENT
None declared.



