-
PDF
- Split View
-
Views
-
Cite
Cite
Todd C. Crawford, Joshua C. Grimm, J. Trent Magruder, R. Scott Stephens, Christopher M. Sciortino, A. Jason Vaught, Janyne Althaus, Ashish S. Shah, Bo S. Kim, A curious case of acute respiratory distress syndrome, Journal of Surgical Case Reports, Volume 2015, Issue 11, November 2015, rjv140, https://doi.org/10.1093/jscr/rjv140
Close - Share Icon Share
Abstract
Gestational acute respiratory distress syndrome (ARDS) is a complicated problem with the potential to gravely harm both mother and fetus. This case report describes a young woman in her second trimester of pregnancy who developed progressive respiratory failure in the setting of newly diagnosed influenza, diffuse alveolar hemorrhage and lymphangioleiomyomatosis. The patient's condition was refractory to conventional interventions and required extracorporeal membrane oxygenation (ECMO) support. Her course was further complicated by preeclampsia requiring preterm delivery with cesarean section while on ECMO. Through novel therapies and a multidisciplinary approach to care, both the patient and her child would overcome these unique and challenging conditions and survive.
INTRODUCTION
Gestational acute respiratory distress syndrome (ARDS) has an estimated incidence of 16–70 per 100 000 pregnancies with a mortality rate approaching 50% [1]. Here, we present a case of a woman in her 21st week of pregnancy who developed severe ARDS with multiple etiologic confounders including lymphangioleiomyomatosis (LAM), influenza, diffuse alveolar hemorrhage (DAH) and preeclampsia. The patient progressed beyond the supportive capabilities of conventional interventions and ultimately required extracorporeal membrane oxygenation (ECMO) support. Fortunately, utilization of novel therapies and timely interventions by multidisciplinary teams enabled this complicated and critically ill pregnant patient and her baby to survive.
CASE REPORT
A 37-year-old, primigravid woman at 21 weeks gestational age presented to a local hospital complaining of fever, cough and progressive shortness of breath. She was initially treated for suspected pneumonia but decompensated rapidly requiring emergent intubation for profound hypoxemia. Given the complexity of her illness, she was transferred to our tertiary care facility.
Upon admission, the patient's chest x-ray (CXR) was notable for bilateral interstitial infiltrates, and she was therefore started on empiric broad-spectrum antibiotics and oseltamivir. She was ventilated with lung-protective settings and paralyzed to alleviate ventilator dyssynchrony. Bronchoscopy revealed DAH and bronchioalveolar lavage cultures yielded Influenza A infection. She was started on high-dose methylprednisolone for her DAH, and inhaled nitric oxide (iNO) was added to optimize oxygenation. After a transient period of improvement, new infiltrates were appreciated on CXR, prompting computed tomography (CT) of her chest. This revealed multiple cysts throughout the lung parenchyma, suspicious for the diagnosis of LAM (Fig. 1).
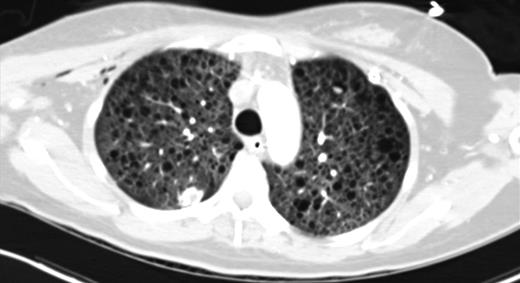
CT chest (axial slices in lung window) demonstrating innumerable cysts scattered throughout all lung fields without basal/apical predilection or cardiophrenic sparing.
The patient's hypoxemia and DAH worsened, and she ultimately required venovenous ECMO support on Hospital Day 15. Though oxygenation improved, DAH remained refractory to steroid therapy. Six days into her ECMO course, recombinant activated factor VII (FVIIa) was endobronchially instilled. Serial bronchoscopies demonstrated interval improvement in the hemorrhagic burden after three treatments of FVIIa.
Ten days after commencing ECMO and at 24 weeks gestational age, the patient developed preeclampsia and HELLP syndrome. The maternal fetal medicine team performed an urgent cesarean section, and a viable female neonate was delivered. Simultaneously, a right-sided wedge biopsy of the lung was performed, and based on histologic findings, the diagnosis of LAM was confirmed (Fig. 2a–d). On ECMO Day 14 and Post-operative Day 3 from cesarean delivery, the patient was successfully decannulated from the ECMO circuit. She was extubated to high-flow nasal cannula (HFNC) 2 days later. Over the course of 2 weeks, she was successfully weaned to room air. The neonate required significant ventilatory and nutritional support but was eventually discharged home without neurologic disability.
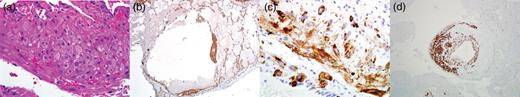
Histologic images from lung biopsy demonstrating perivascular leukocytic infiltrate (H&E 400×) (a), staining for smooth muscle actin (SMA 40×) (b), Hmb-45 (400×) (c) and desmin (100×) (d).
Since discharge from our institution, the patient remains independent of oxygen supplementation. Due to a severe perturbation in her diffusing capacity of the lung for carbon monoxide (DLCO), she was started on sirolimus, a treatment for LAM. Her most recent pulmonary function testing (PFT) revealed a DLCO of 41% predicted and a 6-min walk culminated in desaturation from baseline pulse oximetry of 98–80%. A representative section from her most recent chest CT, performed 8 months after discharge, is shown in Fig. 3.
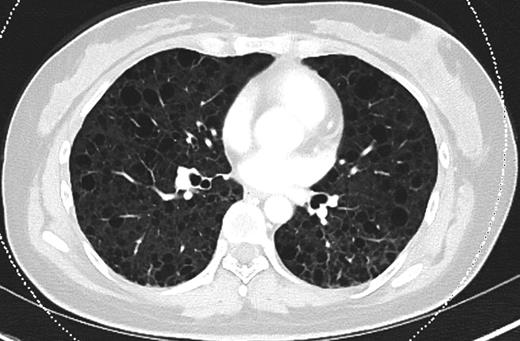
CT chest (axial slices in lung window) demonstrating interval resolution of pneumothoraces and persistent, diffuse numerous thin-walled pulmonary cysts without cardiophrenic sparing.
DISCUSSION
In pregnant patients with ARDS unresponsive to conventional interventions, consideration may be given for ECMO support. Limited data exist regarding the effectiveness of ECMO during pregnancy; however, the H1N1 influenza outbreak of 2009 revealed maternal survival rates of >60% and neonatal survival as high as 70% with extracorporeal support [2].
ECMO cannulation is complicated in the pregnant patient due to fetal inferior vena cava compression, which may impede venous drainage if femoral cannulation is required [2]. Due to anatomic limitations in our patient, bicaval dual lumen cannulation through the neck was not feasible. Thus, inflow cannulation was performed in the right internal jugular vein, and outflow cannulation was achieved in the right femoral vein. Initially, intermittent increases in intrathoracic pressure due to coughing exacerbated her recirculation fraction and compromised systemic oxygen delivery, but this subsided with resolution of alveolar hemorrhage.
LAM is a rare and progressive systemic disease that involves smooth muscle infiltration of the lung, culminating in cystic change of lung parenchyma [3]. The diagnosis of LAM during pregnancy is associated with significantly worse maternal outcomes including an increased incidence of premature delivery [4]. Histologic studies of LAM have demonstrated variable expression of estrogen/progesterone receptors, suggesting a hormonal role in the pathogenesis of LAM [5]. VEGF-D is a serologic marker for LAM, and levels >800 pg/ml have been shown to have 100% specificity [6]. Tissue staining for smooth muscle actin, desmin and HMB-45 is diagnostic for LAM [3]. In a randomized controlled trial, initiation of sirolimus in patients with LAM culminated in preservation of FEV1 and FVC at 1 year compared with placebo; however, this treatment effect was not sustained after cessation of therapy [7]. Limited evidence exists regarding the safety of mTOR inhibitors, specifically sirolimus, during pregnancy.
DAH is a syndrome characterized by injury to the alveolocapillary basement membrane, resulting in red blood cell extravasation into the alveolar spaces. Treatment largely relies on supportive care; however, a small Denmark series demonstrated that local (endobronchial) administration of recombinant activated factor VII (FVIIa) at 50 mcg/kg in 50 cc of normal saline may be beneficial [8]. The therapeutic effect of endobronchial FVIIa draws on its ability to directly bind receptors on the alveolar surface where tissue factor resides in large concentrations.
In conclusion, this is the first reported case of LAM-associated gestational ARDS requiring ECMO. The individual contributions of influenza, DAH, LAM and severe preeclampsia are difficult to quantify in this complicated patient. Endobronchial administration of FVIIa to treat DAH in a pregnant patient with LAM is novel. Most importantly, the initiation of ECMO support was critical to the survival of our patient and her child. Despite its technical challenges, ECMO remains a viable intervention for severe gestational ARDS.
CONFLICT OF INTEREST STATEMENT
None declared.



