-
PDF
- Split View
-
Views
-
Cite
Cite
Adam Hussein, Rohit Makhija, Extensive portal venous gas in a post-operative patient with no identifiable cause, Journal of Surgical Case Reports, Volume 2015, Issue 10, October 2015, rjv136, https://doi.org/10.1093/jscr/rjv136
Close - Share Icon Share
Abstract
Gas within the portal venous system is often considered a pre-morbid radiological sign. We present a case of extensive portal venous gas (PVG) identified in a patient 6 days following emergency Hartmann's procedure for large bowel obstruction. The patient underwent re-laparotomy on the basis of these radiological findings, but no clear cause was identified. She went on to have an uneventful recovery. Of interest is the discrepancy between the extent of PVG on the preoperative imaging in comparison with the lack of positive findings on direct visualization at laparotomy. We discuss the causes of PVG, its clinical significance, strategies for its management and, in particular, whether surgical management is always indicated in such patients.
INTRODUCTION
Portal venous gas (PVG) is a rare radiological sign that has long been considered a marker of high mortality risk. Early studies demonstrated bowel ischaemia as the commonest cause and an associated mortality of 75% [1]. However, these studies largely used plain radiographs for diagnosis. Computed tomography has taken over as the imaging modality most likely to demonstrate PVG—with an increase in reported cases, a wide range of aetiologies and a lower mortality rate have been demonstrated [2]. We present a case of PVG in a post-operative patient, which is unusual in two respects. Firstly, the large amount of gas within the portal venous system, providing interesting radiological images for review. And secondly, the disparity between the radiological findings and operative findings at subsequent re-laparotomy.
CASE REPORT
Our patient, a 64-year-old female, presented acutely with large bowel obstruction. The only past medical history was of gallstone disease treated with laparoscopic cholecystectomy in previous 5 years.
Computed tomography (CT) confirmed obstruction at the level of the proximal sigmoid colon. Although sigmoid diverticular disease was present, the radiological features were more suggestive of malignant aetiology.
Subsequent laparotomy revealed an obstructing sigmoid mass densely adherent to pelvic wall and bladder. The proximal bowel was grossly dilated but viable with no evidence of ischaemia. Hartmann's procedure was performed with en bloc partial cystectomy. The procedure was uncomplicated with minimal blood loss. The dilated proximal colon was decompressed prior to closure, and a tube drain was left in the pelvis.
Post-operatively, she was observed in the high dependency unit (HDU) for 24 h and required no cardiovascular or respiratory support. After step down to the ward, her recovery continued to be uncomplicated. By the end of Day 4, the stoma was working, oral intake was increasing, pain was well controlled with oral analgesia, the pelvic drain had been removed and mobility was improving.
On the fifth post-operative day, her white cell count (WCC) increased to 19.2 (from 12.9 the previous day). On examination, she was borderline tachycardic (heart rate (HR) 90–100, sinus rhythm) with all other observations within normal range, including temperature. There was a superficial wound infection, but her abdomen was soft and non-tender. The wound was opened, washed out and packed on the ward. A full septic screen was requested.
The following day, there was a further increase in the WCC to 21.5, accompanied by an increased CRP to 203 (from 94 the previous day). The patient reported some nausea but no increase in abdominal pain. On examination, the abdomen was slightly distended, but there was no increased tenderness. With no obvious alternative source, a CT scan was requested to exclude intra-abdominal sepsis.
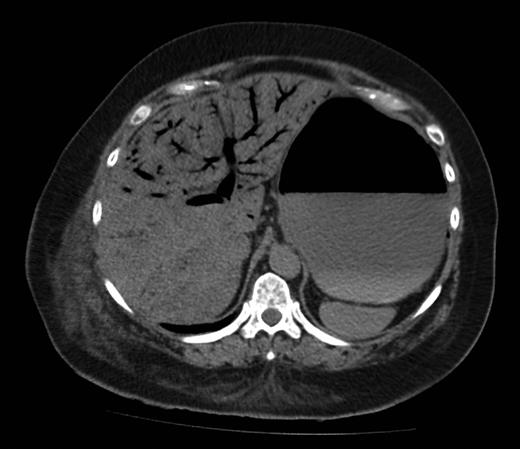
Figure 1 shows a remarkable amount of air in the hepatic portal venous system. Figure 2 demonstrates how the portal vein, splenic vein, superior mesenteric vein and its branches were all outlined with air. Figure 3 shows air within the wall of both small and large bowels. Overall, the radiological findings were suggestive of extensive bowel ischaemia.
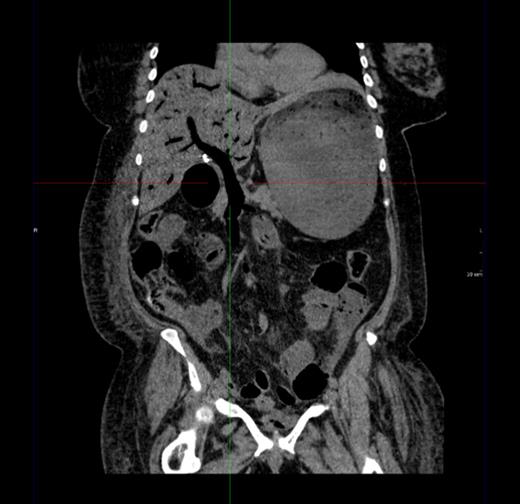
Coronal image showing air outlining the portal vein and superior mesenteric vein.
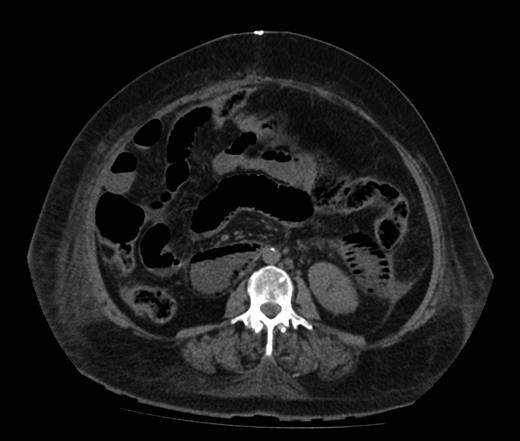
Axial image demonstrating intra-mural gas affecting both small and large bowels.
On clinical review following the CT, the patient appeared stable. She complained of nausea and had vomited ∼500 ml of bilious fluid. There was, however, no reported increase in abdominal pain, and, despite the worrying radiological features, observations and blood gas analysis were surprisingly normal: blood pressure 130/80, HR 97, apyrexial, pH 7.427 and base excess −2.4.
The patient was resuscitated with high flow oxygen, intravenous fluids and broad-spectrum antibiotics. The appropriateness of emergency re-laparotomy was debated. Given the relatively normal haemodynamic and metabolic parameters, it was felt that re-laparotomy for diagnosis and potential treatment was indicated and should be performed as early as possible, prior to physiological deterioration.
Findings at re-laparotomy were unexpected; no evidence of gastrointestinal ischaemia, no intra-abdominal contamination and no other abnormality detected. The patient remained stable throughout the procedure and was extubated post-operatively and taken back to HDU for observation.
Over the following 5 days, she made steady progress without further incident. Her bloods normalized following a course of antibiotics for urinary tract infection. A normal cystogram was performed prior to removal of urinary catheter, and she was discharged with district nurse input for wound dressing. Histological analysis revealed the sigmoid mass to be benign, and she has since made a complete recovery.
DISCUSSION
PVG has traditionally been associated with fulminant bowel ischaemia. More recently, it has been attributed to a variety of reversible, non-life-threatening causes [2]. As such, conservative management has been suggested as an alternative to laparotomy in these patients [2, 3]. However, reported non-fatal cases of PVG that have been managed conservatively have been those where the radiological features are more subtle [3–5]. Our case is extremely unusual in terms of the extent of PVG and the absence of a clearly identifiable cause.
Gastric dilatation has been postulated as a potential cause of PVG [6] and was present in this case. However, this would not explain the presence of air in the superior mesenteric vein and its tributaries. Another potential aetiology is paralytic ileus [7]. Our patient did experience nausea and vomiting at the time of diagnosis; however, this was not on the background of protracted ileus and the small bowel was not grossly dilated at re-laparotomy. The cause of PVG in our case remains unclear.
Our patient underwent re-laparotomy largely on the basis of the radiological signs. Clinical features and laboratory results were unchanged and not consistent with extensive bowel ischaemia. Where there is a discord between the radiological signs and the overall clinical picture, a conservative approach has been advocated [3, 8]. In hindsight, expectant management of this patient with avoidance of negative laparotomy may have been preferable.
In conclusion, this case demonstrates that extensive PVG is not necessarily a pre-morbid sign. A definite aetiology for PVG is not always identified. The overall clinical picture should be used to decide upon surgical versus conservative management. Expectant management is a valid option even in the presence of large volumes of PVG.
CONFLICT OF INTEREST STATEMENT
None declared.



