-
PDF
- Split View
-
Views
-
Cite
Cite
I. Balasundaram, I. Al-Hadad, K. Rehman, I. McCafferty, A. Monaghan, The use of foam sclerotherapy to treat low-flow vascular malformations of the head and neck, Journal of Surgical Case Reports, Volume 2014, Issue 9, September 2014, rju095, https://doi.org/10.1093/jscr/rju095
Close - Share Icon Share
Abstract
Liquid sclerotherapy, laser and surgery have been used in the treatment of head and neck vascular anomalies with variable success for many years. A multidisciplinary team consisting of plastic surgery, maxillofacial surgery and interventional radiology currently treats such lesions by converting liquid sclerosant into foam. Foam sclerotherapy is currently used successfully to treat varicosities of the lower limbs and in this study, we present four cases in which 3% sodium tetradecyl sulfate has been used to treat low-flow vascular malformations in the head and neck.
INTRODUCTION
Historically, the classification of vascular anomalies has been inconsistent leading to inaccurate diagnosis and inappropriate treatment. In 1982, Mulliken and Glowacki [1] introduced a biological classification for head and neck vascular anomalies according to clinical presentation, behavior and histology. There were two main categories, hemangiomas and vascular malformations, which were revised later to vascular tumors and vascular malformations as summarized in Table 1 (http://emedicine.medscape.com/article/846692-overview, July 2014). Vascular malformations are further split into high- and low-flow types.
| Vascular tumors . | Vascular malformations . |
|---|---|
| Hemangioma | High flow |
| Kaposiform hemangioendothelioma | Arteriovenous malformation |
| Tufted angioma | Low flowa |
| Capillary | |
| Lymphatic | |
| Venous |
| Vascular tumors . | Vascular malformations . |
|---|---|
| Hemangioma | High flow |
| Kaposiform hemangioendothelioma | Arteriovenous malformation |
| Tufted angioma | Low flowa |
| Capillary | |
| Lymphatic | |
| Venous |
aLesions treated at University Hospital Birmingham Foundation NHS Trust with foam sclerotherapy.
| Vascular tumors . | Vascular malformations . |
|---|---|
| Hemangioma | High flow |
| Kaposiform hemangioendothelioma | Arteriovenous malformation |
| Tufted angioma | Low flowa |
| Capillary | |
| Lymphatic | |
| Venous |
| Vascular tumors . | Vascular malformations . |
|---|---|
| Hemangioma | High flow |
| Kaposiform hemangioendothelioma | Arteriovenous malformation |
| Tufted angioma | Low flowa |
| Capillary | |
| Lymphatic | |
| Venous |
aLesions treated at University Hospital Birmingham Foundation NHS Trust with foam sclerotherapy.
Low-flow vascular malformations (LFVM) include venous, capillary and lymphatic forms and are present at birth, never involute, remain throughout life and tend to grow. Current treatments include laser, surgery and sclerosing agents.
Currently, the University Hospital Birmingham Foundation NHS trust is the one of the few centers in the UK offering foam sclerotherapy treatment for LFVM in the head and neck. Patients had been referred from a national base from general practice, otolaryngology, dermatology, neurology, maxillofacial/oral surgery and plastic surgery. The procedure is done by interventional radiology as a day case in theater under general anesthetic or regional block. Foamed 3% sodium tetradecyl sulfate (STD) is used. Four cases are presented below, of patients who have received foam sclerotherapy for LFVM.
CASE REPORTS
Case 1
Case 1 is a 25-year-old male with a venous malformation on the left cheek. His main concern was the visible deformity. The treatment was completed as a day case. The patient did not suffer from any serious complications. Following treatment the patient claimed his self-confidence, looks, smile and his ability to eat has improved. The patient found the treatment very successful and requires no further interventions Fig. 1.

Case 2
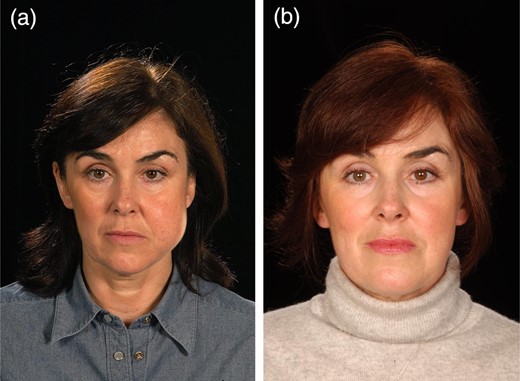
Case 2 is a 47-year-old male with a venous malformation of the right cheek. His main concern was the pain caused by the lesion and the visible deformity. The patient suffered no serious complications following treatment. The patient had the treatment to improve his self-confidence and relieve the pain which has been achieved. The patient has further foam injections planned (Fig. 2).
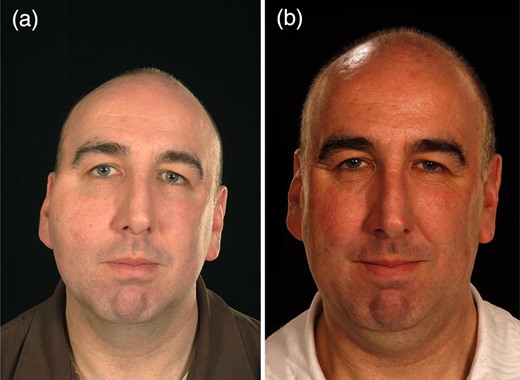
Case 3
Case 3 is a 52-year-old female with a venous malformation over her right cheek and tongue. Her main concerns were the deformity caused by the lesion. She had no complications following treatment. The patient felt her confidence, appearance, smile, social life, ability to eat and speech improved. She reports that the swelling has completely resolved, and that the treatment has been very successful (Fig. 3).
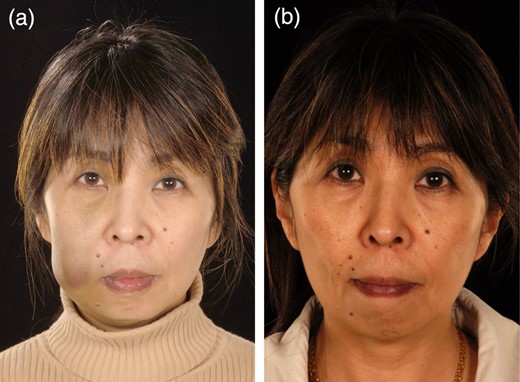
Case 4
Case 4 is a 54-year-old female with a venous malformation over her left cheek. Her main concerns were the deformity and pain caused by the lesion. She had no complications following treatment. The patient felt her confidence, appearance, smile and social life improved. She reports that the swelling has completely resolved, and that the treatment has been very successful (Fig. 4).
DISCUSSION
Liquid sclerosants have been used for many years in the treatment of LFVMs, but its use is limited as the agent is diluted within the circulation and becomes less active. It gets irregularly distributed over the endothelium reducing its effectiveness, and controlling its distribution within the circulation is difficult, increasing the risks of systemic side effects. Tessari et al. [2] presented a three-way tap technique to produce a microfoam simply using a three-way tap and two syringes. Converting liquid sclerosant to foam improves the procedure as foam displaces the blood instead of diluting itself and distributes more evenly over the endothelium. The increased surface area created by the bubbles allows larger lesions to be treated. The foam can also be visualized with ultrasound, and therefore distribution within the circulation can be better controlled [3].
We did not document any serious adverse events, such as neurotoxicity or evidence of embolism. Minor adverse events such as bruising, swelling and lumpiness were reported in the majority of patients, but this is expected as part of the inflammatory response to the treatment.
Ethanol is more potent than STD; however, our department opted not to use ethanol due to its toxicity. Berenguer et al. [4] reported that it is mandatory to carefully monitor patients during ethanol sclerotherapy due to previous evidence of cardiopulmonary collapse. Patients will need general anesthesia as the injection of ethanol is very painful, and De Lorimer [5] claimed that the dose used needs to be limited as cerebral intoxication can occur with small amounts. Siniluoto et al. [6] stated that, despite better success rates with ethanol, they still use STD as the primary sclerosant when treating venous malformations, but will use ethanol where it fails.
With regard to other treatments available, Pappas et al. [7] have stated that ‘complete surgical eradication of extensive cervical facial venous vascular malformation is rarely possible without jeopardizing function or risking additional disfigurement’. Regarding the use of laser, Yakes noted that it is only useful in superficial lesions and can result in significant scarring [4].
This case series has demonstrated that foam sclerotherapy can successfully be used to treat LFVM in the head and neck primarily, or as an adjunct prior to surgery. However, this series was limited in the small numbers of patients who have been treated to date. Follow-up with more patients as well as long-term follow-up of existing patients is needed. The National Institute of Clinical Excellence (UK) advocated the use of foam sclerosants to treat varicose veins in the lower limbs, and they advise that outcomes need to be closely audited (http://www.nice.org.uk/guidance/IPG440/chapter/1-guidance, July 2014). Likewise, we feel that the use of foam sclerotherapy in the head and neck should be managed as part of a multidisciplinary team limited to specialist centers, and auditing outcomes of patients is essential.
CONFLICT OF INTEREST
None declared.



