-
PDF
- Split View
-
Views
-
Cite
Cite
Logan R. Mckenna, Edward L. Jones, Teresa S. Jones, Trevor Nydam, Csaba Gajdos, Recurrent intravenous leiomyosarcoma of the uterus in the retrohepatic vena cava, Journal of Surgical Case Reports, Volume 2014, Issue 9, September 2014, rju090, https://doi.org/10.1093/jscr/rju090
Close - Share Icon Share
Abstract
Although intravenous extension of uterine leiomyosarcomas has been described, extension into the inferior vena cava (IVC) and right atrium, so-called ‘intravenous leiomyosarcomatosis (IVLS)’, is rare. To our knowledge only a few cases have been described in the literature. We describe a case of recurrent uterine leiomyosarcoma to the retrohepatic IVC. The patient was initially treated with total abdominal hysterectomy. Follow-up computed tomography a year later showed an extensive intravascular and intracardiac soft tissue mass treated with tumor extraction using cardiac bypass. Five years later she presented to our institution with a new retrohepatic caval mass treated with surgical resection and caval grafting. IVLS is a rare disease that is best treated with surgical resection even in the recurrent setting. The role of adjuvant therapy remains unclear.
INTRODUCTION
Although uterine intravenous leiomyomatosis (IVL) is a well-described process, intravenous extension of uterine leiomyosarcoma (LMS) into the inferior vena cava (IVC) and heart is rare. To our knowledge, this has only been described previously in a few case reports [1–3]. We describe a rare case of recurrent uterine LMS to the retrohepatic IVC after resection of a prior intravascular and intracardiac tumor extension.
CASE REPORT
A 40-year-old woman was diagnosed with uterine fibroids in 2006. Her symptoms failed to improve and she subsequently underwent a total abdominal hysterectomy. Pathology at that time showed a benign leiomyoma. The following year, the patient experienced recurrent palpitations and new bilateral lower extremity swelling. Computed tomography (CT) identified a soft tissue mass extending from the left ovarian vein through the left renal vein into the IVC and right atrium. The patient underwent tumor thrombectomy through a venotomy in the abdominal IVC (done via laparotomy), as well as intracardiac tumor excision via a median sternotomy requiring cardiopulmonary bypass. Pathology demonstrated a low-grade LMS with a positive caudal margin in the intravascular specimen. She recovered well and was treated with an anti-hormonal agent for 6 months.
The patient was followed with serial imaging for the next 6 years when a recurrent mass was noted in the retrohepatic IVC. The tumor originated just above the renal veins and extended into the hepatic veins (Fig. 1). At this point she was referred to the University of Colorado for further evaluation. Her work-up included CT angiogram and an magnetic resonance imaging (MRI) of her liver. The CT showed a 4 cm by 3 cm intracaval mass at the level of the IVC-hepatic vein confluence. An additional 9-mm nodular mass was noted within the cava raising the possibility of dis-contiguous disease (Fig. 1). Following appropriate preoperative counseling, the patient was taken to the operating room for surgical resection. Extensive lysis of adhesions was performed from a large midline incision. Following complete mobilization of the liver, and control of the suprahepatic and infrahepatic cava, she underwent segmental resection of the IVC between the renal and hepatic veins with reconstruction using a 24-mm Dacron tube graft. All grossly visible tumor was removed (Fig. 2).
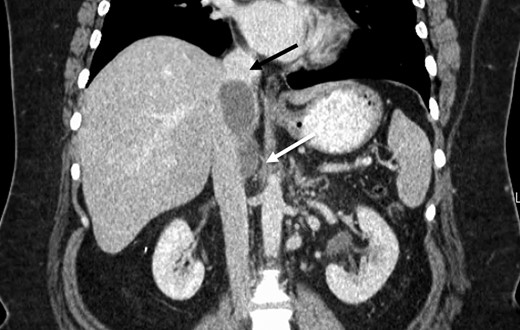
Preoperative CT identifying a smooth, well-circumscribed mass within the IVC arising superior to the right renal vein long (white arrow) and terminating just prior to right hepatic take-off (black arrow).
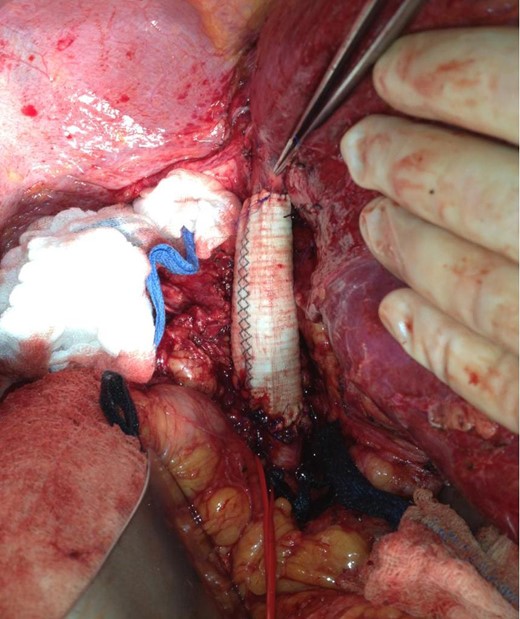
Extensively mobilized liver allows excellent view of the completed IVC reconstruction with Dacron interposition graft. Red vascular loop surrounds the right renal vein and forceps indicate take-off of the right hepatic vein.
The patient recovered uneventfully and was discharged home. Pathology demonstrated a low-grade LMS with negative margins. She has returned for follow-up and is doing well. She resumed surveillance imaging showing stable IVC wall thickening at 17 months postoperatively (Fig. 3). No adjuvant therapy was administered.
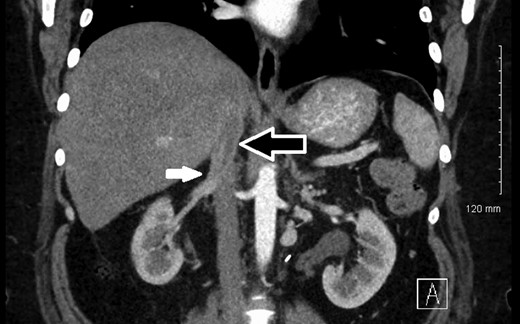
12-month postoperative CT showing stable IVC thickening (white arrow) after segmental resection of the IVC with tube graft reconstruction (black arrow).
DISCUSSION
Intravascular extension of abdominal tumors into the IVC is rare. It is most frequently seen in renal cell carcinomas, but it has also been described in uterine neoplasms, hepatocellular carcinoma, adrenocortical carcinoma and retroperitoneal sarcomas [4, 5]. IVL is a well-described variant of uterine leiomyoma involving striking intravascular extension which can extend into the IVC via the uterine and iliac veins and even reach the right heart. However, this pattern of vascular involvement from an LMS is extremely rare with only a few previous reports in the literature [1–3]. The term intravenous leiomyosarcomatosis (IVLS) has been proposed to designate this phenomenon [1].
Patients can present with symptoms from their uterine tumor, including irregular vaginal bleeding, abdominal discomfort or a palpable pelvic/abdominal mass. Depending on the extent of tumor, patients may also present with symptoms ranging from lower extremity edema to palpitations, dyspnea and congestive heart failure due to tumor extension into the right atrium. Pulmonary embolism from the tumor is rare, but has been previously described [3].
High-resolution CT/computed tomography angiography or MRI/magnetic resonance angiography should be used to define the size of the mass and the extent of vascular involvement. Depending on the extent of vascular and intracardiac involvement, the patient may require cardiopulmonary bypass or even circulatory arrest to achieve complete resection [2]. The final diagnosis is usually made by biopsy of the uterine mass, although endovascular biopsy of the intravascular tumor component has been performed successfully with radiologic guidance [2, 3].
Uterine LMS remains a heterogeneous group of tumors with a wide variety of clinical outcomes. True uterine LMS is an aggressive disease and carries a poor prognosis with a 15–25% 5-year survival [6]. However, a subset of these patients may experience a protracted clinical course as did our patient. Thus, the diagnosis of ‘low-grade’ uterine LMS had been proposed but remains controversial [7].
No treatment guidelines for IVLS exist due to the rare nature of this disease. However, it may be useful to extrapolate from other malignancies. In renal cell carcinoma, where intracaval tumor extension is most common, concurrent resection of the caval disease is the standard of care [4, 5]. Surgical resection improves intravascular obstructive symptoms, decreases the risk of tumor embolus, and may offer a survival benefit [4, 5].
Surgical resection of IVLS should be recommended on a case-by-case basis by a multidisciplinary team. However, the role of chemotherapy, radiation and hormonal therapy is unknown and long-term data are lacking. Therefore, early surgical resection and follow-up with serial imaging remain the primary treatment modality for IVLS.
The complexity of these surgeries comes from tumor location. Proximal extension of the tumor can involve the junction of hepatic veins and the IVC. Obtaining negative proximal margins may require caval resection extending above the origin of the hepatic veins. Various methods of reconstruction, including hepatic vein re-implantation into a tube graft, have been described [8]. Also, large case series involving reconstruction of the IVC in the setting of cancer are lacking and the long-term performance of these grafts is unknown. In one small case series of 17 patients with renal cell cancer who underwent graft reconstruction of the IVC, 2 patients (11%) developed graft thrombosis within 18 months [9].
In summary, recurrent intracaval LMS can be successfully treated with surgical resection. Patients should be evaluated by a multidisciplinary team with high-quality imaging to determine the exact extent of disease. Replacement of the involved IVC with Dacron tube graft can be performed successfully. Medical therapies are unproven and should be used if surgical treatment is not possible.



