-
PDF
- Split View
-
Views
-
Cite
Cite
Meera Gangadharan, Sanjeet Panda, P. Stephen Almond, Vaidehi Agrawal, Angelina Bhandari, A. Jay Koska, Management of preterm giant sacrococcygeal teratoma (GSCT) with an excellent outcome, Journal of Surgical Case Reports, Volume 2014, Issue 12, 1 December 2014, rju132, https://doi.org/10.1093/jscr/rju132
Close - Share Icon Share
Abstract
Infants born with a giant sacrococcygeal teratoma (GSCT; >10 cm) have high mortality. Risk factors for mortality include increased tumor vascularity, high cardiac output, rapid growth, diagnosis before 20-week gestation, delivery before 30-week gestation, hydrops, low birth weight, Apgar less than 7 at 5 min and polyhydramnios. We present the case of a 28-week infant born with a GSCT (15 × 12 × 16 cm) and all of these risk factors.
INTRODUCTION
Giant sacroccocygeal teratomas (GSCTs) are rare tumors derived from the ectoderm, mesoderm and endoderm, and are associated with high infant mortality [1]. Infants may be diagnosed and/or treated antenatally or postnatally. GSCT infants with high-output cardiac failure and hydrops may be managed antenatally [2]. Antenatal interventions include fetoscopic laser ablation, radiofrequency ablation and interstitial laser ablation of blood flow to the tumor. The use of these interventions is associated with infant survival rates of 30–50%. Similar survival rates are noted in GSCT infants with high-output cardiac failure, rapidly growing tumors, and solid and highly vascularized teratomas, managed with traditional intervention methods [2]. We cared for an infant diagnosed antenatally with a GSCT. Antenatal treatment included packed red cell transfusions and aminoreductions. Postnatal treatment included resuscitation, correction of coagulopathy and timely resection of the lesion. We report our management approach of this high-risk infant.
CASE REPORT
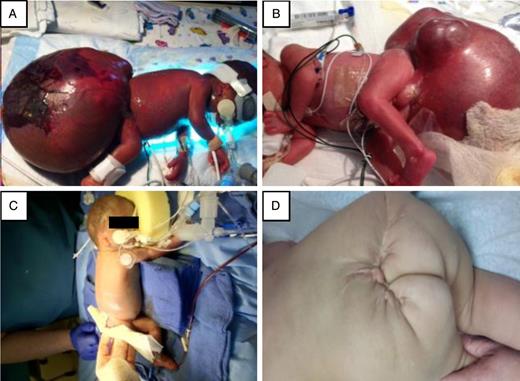
(A) At Day 0, a GSCT (15 × 12 × 16 cm) is noted with skin necrosis on the left decubitus position. (B) Irregular multinodular contour noted on GSCT on the right decubitus position. (C) Infant on post operative day 0 after GCST resection. (D) Infant at 15 month follow up.
At operation, a transverse infra-umbilical incision was made and the aorta was dissected and looped for vascular control. He was then turned prone and the tumor resected via a chevron incision (Fig. 1C). The procedure was stopped multiple times to volume resuscitate the child. Post resection, the retrorectal space was packed with thrombin and gelfoam, the skin flaps trimmed and the wound closed. Estimated blood loss was 400 ml and he received 360 ml of PRBCs, 60 ml of platelets, 120 ml of FFP and 500 ml of lactated ringers solution. Postoperative laboratory values showed pH 7.15, pCO2 71, pO2 285, base deficit 6.6 and a lactate of 10 mM. Fibrinogen was 93 mg/dl, prothrombin time 19.4, international normalized ratio 1.8, hematocrit 38.1 and platelets 34 000. The infant returned to the operating room on postoperative Day 5 for wound debridement. Pre- and postoperative head ultrasounds were normal. The patient was discharged 3.5 months later (42 weeks post conception).
Pathology confirmed a GSCT with high endodermal sinus component and potential malignant foci. At 2.5, 5 and 8 months, the AFP dropped from 1444 to 67.2 and 8 ng/ml, respectively. Oncology recommended follow-up every 3–6 months. At 18 months of age, the patient is at the 10th percentile for weight and length (Fig. 1D). He has chronic lung disease and systemic hypertension, which is managed with enalapril. He continues having physical therapy to improve the strength of his lower extremities.
DISCUSSION
SCT occurs in 1 out of every 40 000 live births with a female preponderance [1]. SCTs are classified into three prognostic groups: (A) tumor diameter <10 cm, absent or mild vascularity, and slow growth; (B) diameter >10 cm with pronounced vascularity, high-output cardiac failure and rapid growth; (C) diameter of 10 cm or greater, predominantly cystic with absent or mild vascularity and slow growth [2]. Our patient was in Group B. Group B infants have an overall mortality of 50%, but if hydrops is present, mortality approaches 100%. Additional poor prognostic risk factors are diagnosis before 20-week gestation, delivery before 30-week gestation, development of hydrops, low birth weight, Apgar less than seven at 5 min and presence of polyhydramnios. Our patient was diagnosed at <20 weeks gestation and delivered at 27 weeks. In the absence of fulminant hydrops, pre-emptive early delivery in high-risk SCT at 27–32 weeks gestation is associated with good outcomes [2].
We optimized our patient prior to tumor resection. Tumor rupture, tumor lysis with hyperkalemia and hemorrhage may force emergent resection with variable outcomes [3]. Our infant had pulmonary hypertension that failed conventional treatment methods, requiring nitric oxide.
Blood loss is a primary cause of mortality in GSCT infants. Techniques to decrease blood loss include laparoscopic ligation or angiographic embolization of feeding vessels and utilization of extracorporeal membranous oxygenation (ECMO) [3 –5]. In our case, no large feeding vessels were identified and our infant was preterm, precluding the use of ECMO and angiographic embolization. We used a technique described previously [5]. Our 700 g infant (weight with tumor was 2.7 kg) lost 400 ml of blood (3 blood volumes). We administered 360 ml of AS-3 PRBC and a transfusion ratio of 6 : 2 : 1 with no transfusion-associated complications.
Neonatal massive transfusion protocols (nMTPs) have not yet been established. The Children's National Medical Center is currently evaluating FFP, platelets and PRBCs at a 1 : 4 : 1 unit ratio [6]. In pediatric liver transplants, transfusion of >10 U of FFP has been associated with multiorgan failure [7]. While the safety of AS-3 PRBCs in massive transfusions for sick premature neonates has not been established, small volume transfusions have been demonstrated to be safe [8]. Autologous cord blood was successfully used in a single-term infant with GSCT in Japan [9].
Pathology confirmed a GSCT. As 10–20% of these recur in the first 3 years of life and 40% become malignant, our patient is being closely monitored [10].
Overall, our report shows that premature infants with GSCT with heart failure and hydrops can be managed successfully. This requires interdisciplinary prenatal, postnatal and perioperative management.
CONFLICT OF INTEREST STATEMENT
All informed consents are available upon request to the corresponding author. The article has been not been published nor been submitted simultaneously elsewhere for publication. The authors have no conflicts of interest to report.



