-
PDF
- Split View
-
Views
-
Cite
Cite
Divya Dahiya, Sanjay Bhadada, Uma Nahar, Mandeep Garg, Lileswar Kaman, Arunanshu Behera, IGF-II-secreting pelvic tumor presenting with neuropsychiatric symptoms, Journal of Surgical Case Reports, Volume 2013, Issue 12, December 2013, rjt116, https://doi.org/10.1093/jscr/rjt116
Close - Share Icon Share
Abstract
A 48-year-male presented with a gradually increasing lump in the abdomen, which was confirmed on investigations as a pelvic tumor. He developed frequent episodes of hypoglycemia and neuropsychiatric symptoms while awaiting surgery. During episodes of hypoglycemia, his serum C-peptide and insulin levels were negligible and the insulin-like growth factor (IGF)-II to IGF-I ratio was >10. He successfully underwent an en bloc excision of the tumor. Histopathology revealed a solitary fibrous tumor.
INTRODUCTION
Hypoglycemia associated with non-islet cell tumor (NICT) is one of the manifestations of paraneoplastic syndrome. The most common NICTs causing hypoglycemia are mesenchymal tumors. Frequently implicated mesenchymal tumors that cause hypoglycemia are solitary fibrous tumor (SFT), fibrosarcoma, leiomyosarcoma, mesothelioma and hemangiopericytom [1]. High-molecular-weight insulin-like growth factor-II (Big-IGF-II) secretion plays an important role in causation of hypoglycemia [2]. Herein we present the patient with neuroglycopenic symptoms due to IGF-II-secreting SFT.
CASE REPORT
A 48-year-male patient presented with awareness of gradually increasing lower abdominal lump and abdominal discomfort for 8 months. There was no history of anorexia and weight loss. He was a non-smoker and non-alcoholic. His general physical examination was unremarkable with no clinical evidence of hepatic disease and adrenal or pituitary insufficiency. On abdominal examination, about 8 × 10 cm non-tender, hard, well-defined intra-abdominal lump was present in hypogastrium, which was extending into right iliac fossa, right lumbar and umbilical region for which the lower margin was not palpable. The lump was bimanually palpable on rectal examination. It was causing intra-luminal projection within rectum without any mucosal irregularity. His initial basic laboratory investigations which included hemogram, coagulogram, blood sugar, serum sodium, potassium, chloride, calcium, renal and liver function tests and urine analysis were within normal range. CECT abdomen (Figs 1 and 2) revealed a 20 × 15 × 7 cm heterogenous well-defined lobulated mass with calcification and areas of necrosis in the pre-sacral region and was extending superiorly up to the lower border of L3. It was indenting into the bladder anteriorly and rectum posteriorly, also abutting pelvic vessels but maintaining fat planes with all these structures. This mass was causing bilateral hydroureteronephrosis.
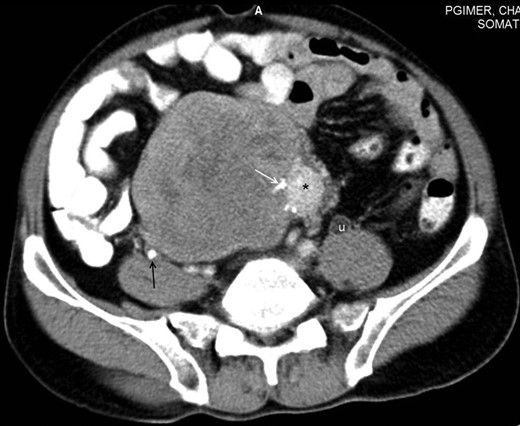
Axial section of contrast-enhanced CT of the abdomen reveals a large heterogeneously enhancing mass with a small hyperenhancing component (asterisk). Foci of calcification are noted within the lesion (white arrow). A few hypoenhancing areas are also seen within the lesion, which might represent necrosis or scar formation. Incidentally, a ureteric calculus is seen on the right side (black arrow). Dilated ureter (u) is noted on the left side due to compression by the mass lower down. Note that the fat planes with the bowel and the retroperitoneal vessels are maintained.
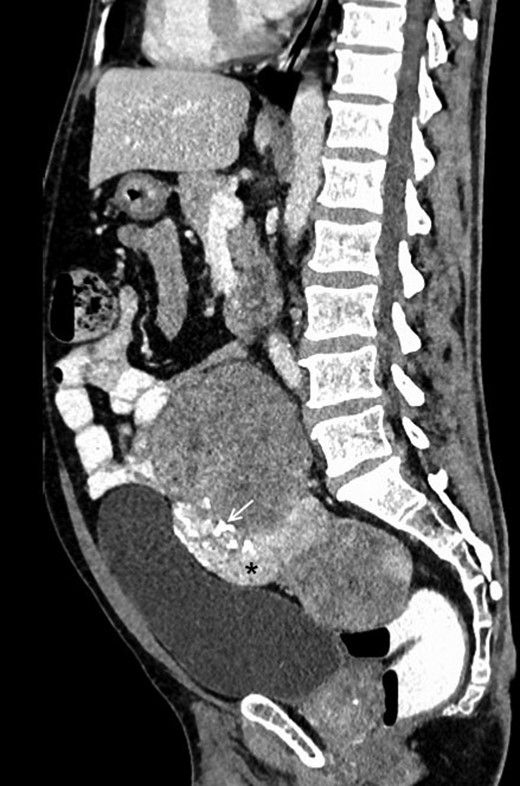
Contrast-enhanced sagittal reformatted CT section of the abdomen depicts a large enhancing mass posterior to the urinary bladder and anterior to the lower lumbar vertebrae and sacrum. The lesion is heterogeneously enhancing with a small hyperenhancing component (asterisk). Foci of calcification are noted within the lesion (white arrow). Note that the fat planes with the bowel and the urinary bladder are maintained.
During his hospital stay while awaiting surgery he displayed a widely fluctuating behavior, slurring of speech, double vision, episodes of aggression and confusion, followed by lucid interval. There was no relation with food and this first episode was self-limiting. He did not give history of any such behavior prior to his visit to the hospital. Fundus examination was normal. CT brain did not reveal any abnormality. Psychiatrist made a diagnosis of delirium of unknown origin and started benzodiazipines for him. After 2 days, he again underwent a similar episode which was also associated with hemiparesis. His blood sugar during this episode was 31 mg% and he responded symptomatically and biochemically after receiving 50 ml 50% dextrose infusion, thus fulfilling the criteria for diagnosing organic hypoglycemia (Whipple's triad). History of diabetes, intake of hypoglycemic or steroids was ruled out. During his next episode of hypoglycemia (blood sugar 30 mg%); C-peptide was 0.709 (1.1–4.4) ng/ml, serum insulin was <0.200 (2.6–24.9) μU/ml and ketones were 0.2 mmol/l. The concentration of IGF II was in the normal range, 623 (288–736) ng/ml, while IGF I was reduced, 56.1 (94–252) ng/ml. But the ratio of IGF II–I was 11.105 (reference range is <10.00), which was highly suggestive of IGF II-secreting NICT. Thyroid function tests, serum cortisol were within the normal range.
He was maintained on 10% dextrose infusion and subsequently was taken up for laparotomy. There was no visceral, peritoneal or lymphatic metastasis. There was a 20 × 13 cm solid well-encapsulated vascular tumor present in the pelvic cavity posterior to urinary bladder, which was extending above and anterior to the right common iliac vessels. An en bloc excision of the tumor was performed (Figs 3 and 4).
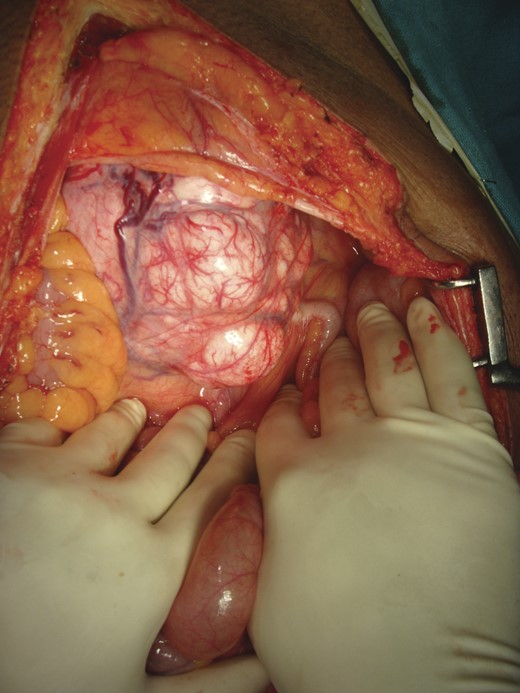
Intra-operative picture: a 20 × 13 cm solid well-encapsulated tumor was present in the pelvic cavity posterior to urinary bladder.
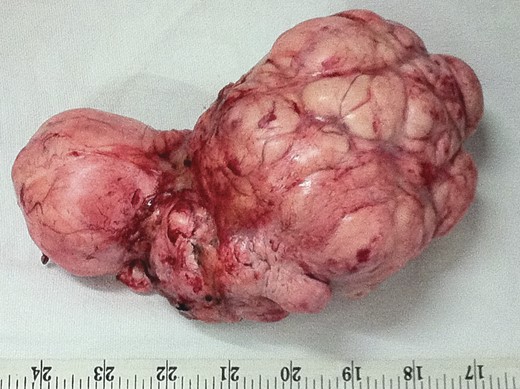
Cut section of tumor was nodular with areas of necrosis, calcification and hemorrhage. Histopathology diagnosis was consistent with SFT with short interlacing spindle cells in short fascicles and with thick intervening collagen (Fig. 5). There was no evidence of mitosis. There were areas of ischemic necrosis and osseous metaplasia. Immunostaining for CD 34 was strongly positive (Fig. 6).
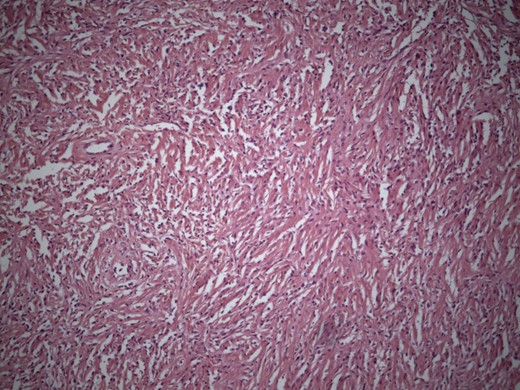
Microphotograph showing short interlacing spindle cells in short fascicles with intervening thick collagen (H&E × 200).
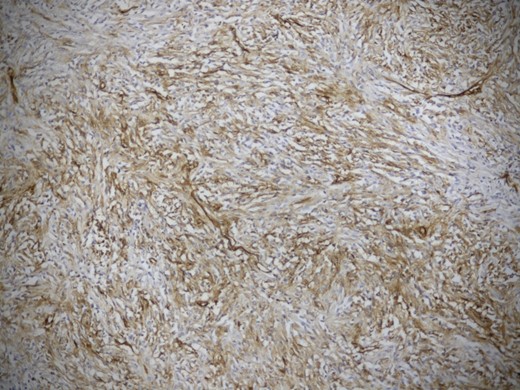
Microphotograph showing a strong CD 34 positivity in the tumor cells (IHC × 200).
The patient had an uneventful postoperative recovery. He did not have any episode of hypoglycemia during his hospital stay after surgery and subsequently at home after discharge. He received radiotherapy to tumor bed postoperatively as an adjuvant treatment. He is doing well on follow-up after 6 months without recurrence of tumor and hypoglycemia.
DISCUSSION
Non-islet cell tumor-induced hypoglycemia (NICTH) is a rare paraneoplastic syndrome and is caused by the overproduction of incompletely processed IGF-II. Hypoglycemia can also be caused by autoantibodies against insulin or insulin receptors, substantial tumor infiltration of liver and adrenal glands, or by excessive utilization of glucose by a large tumor itself. However, the major cause of hypoglycemia is inhibition of gluconeogenesis and increase glucose utilization in skeletal muscles due to Big-IGF-II.
It was first described for hepatocellular carcinoma (HCC) in 1929 [3]. It can occur both in benign as well as in malignant tumors. It has been chiefly reported in solid tumors of mesenchymal or epithelial origin, but it has also been reported in tumors of hematopoietic (myeloma, lymphoma) or neuroendocrine (carcinoid, malignant pheochromocytoma) origin, hemangiopericytomas and colorectal carcinomas [4]. Tumors causing NICTH are either very large or metastatic.
NICTH suppresses insulin secretion, lipolysis and ketogenesis, leading to low serum concentration of C-peptide, growth hormone (GH) and beta-hydroxy butyrate [4]. Diagnosis is confirmed by blood sugar estimation <50 mg%, low or negligible serum C-peptide, insulin and GH, low serum IGF-I, normal or elevated levels of IGF-II [5]. The IGF-II to IGF-I molar ratio >10 is highly suggestive of IGF-II-secreting tumor [6].
Clinical presentation in NICTH may vary from subtle symptoms of hypoglycemia (anxiety, palpitation, sweating, numbness) to severe neuroglycopenic (abnormal mentation, impaired judgement, fatigue, personality changes, confusion, amnesia, delirium, double vision, slurred speech, paralysis, hemiparesis, seizures, amnesia) symptoms [4, 5, 7]. In addition, they may present with acromegaloid features [4, 8]. The patient in our study developed neuropsychiatric symptoms secondary to IGF-II-secreting abdominal SFT without any clinical evidence of acromegaly.
Curative treatment is complete excision of tumor, which leads to complete reversibility of metabolic alterations caused by IGF-II. If removal of tumor is not a possibility then alleviation of hypoglycemia is a challenge. Various options like debulking of tumor, directed chemotherapy, selective tumor embolization, radiotherapy or medical treatment with diazoxide, steroids, octreotide or GHs individually or in combination can be tried to improve the symptoms of hypoglycemia [7].
NICT is one of the major causes of hypoglycemia, which develops as a result of excessive production of Big-IGF II. In a patient with mesenchymal tumor with neuropsychiatric symptoms, NICTH should be considered after ruling out intra-cerebral vascular events and brain metastasis. Tumor resection can lead to complete resolution of symptoms.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest between authors.



