-
PDF
- Split View
-
Views
-
Cite
Cite
Sally Hallam, Ajay Aggarwal, Domonica Predolac, Giles Cunnick, Richard Ashford, Primary ectopic breast carcinoma in a supernumerary breast arising in the anterior chest wall: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2013, Issue 12, December 2013, rjt107, https://doi.org/10.1093/jscr/rjt107
Close - Share Icon Share
Abstract
Accessory mammary tissue occurs in 0.2–6% of women, and is under the same hormonal influences as breast tissue, potentially undergoing malignant transformation. Carcinomas of accessory mammary tissue account for ∼0.3% of breast cancers, 5% of which are within a supernumerary breast. Due to its rarity and the low index of clinical suspicion, it is often diagnosed at a later stage compared with breast cancer. Reports of carcinoma developing in a supernumerary breast are rare. We describe a case of a 48-year-old woman, presenting with ectopic breast carcinoma within a supernumerary breast below the inframammary fold. We describe the mode of presentation, diagnosis and treatment with reference to the available literature. The radiotherapy treatment plan is discussed in detail to provide reference for future cases as the available literature offers no formal guidance on radiotherapy dose, fractionation or treatment field.
INTRODUCTION
Carcinomas of accessory mammary tissue account for ∼0.3% of all breast cancers, 5% of which are within a supernumerary breast [3]. Due to its rarity and the low index of clinical suspicion, late diagnosis is common [2, 6, 7, 9]. The following case report describes a case of ectopic breast carcinoma within a supernumerary breast below the inframammary fold. We describe the mode of presentation, diagnosis and treatment with reference to the available literature.
CASE REPORT
A 48-year-old female presented with a 6-week history of a tender mass inferior to the right inframammary fold following trauma.
Antibiotics were initiated for presumed infection; however, the mass persisted, prompting surgical referral. Clinical examination demonstrated a 2 × 1 cm superficial mass, with adjacent accessory nipple. An ultrasound confirmed a 15-mm hypo-echoic mass consistent with a sebaceous cyst; however, a core biopsy confirmed a Grade 2 invasive ductal carcinoma (IDC), strongly positive for oestrogen and progesterone receptors (Allred scores of ER 7, PR7). Immunohistochemistry for Her 2 status was negative (0).
Magnetic resonance imaging (MRI) confirmed a 22 × 12 mm spiculated (Fig. 1) mass inferior to the right inframammary fold deep to the accessory nipple, compatible with a supernumerary breast. There was evidence of overlying skin infiltration, with a clear margin of normal tissue between the tumour and costal fascia. No masses were noted in either breast.
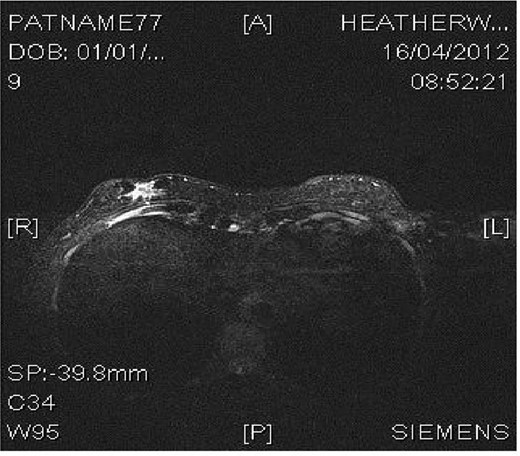
MRI image demonstrating right inframammary breast mass within an accessory breast.
She proceeded to a wide local excision of the entire accessory breast, nipple-areolar complex and an additional ellipse of skin. Sentinel node biopsy was negative, with no further axillary dissection performed.
Histopathology revealed a Grade 2, 19-mm IDC. There was no lymphovascular invasion and radial margins were clear by >10 mm. The deep margin was 2 mm, but the anterior subcutaneous margin was 0 mm. In view of the positive anterior margin the patient proceeded to adjuvant radiotherapy.
The patient underwent a CT planning scan to assess the depth of tissue to be treated. A 10 × 7 cm rectangular electron field was applied to the tumour bed incorporating the excision scar. The patient was then treated with 9 megavoltage (MEV) electrons to a dose of 40 Gy in 15 fractions, over 21 days receiving 2.67 Gy per fraction. The Dmax treatment depth was 12 mm, with the 90% isodose at 18 mm. The ipsilateral breast was not included in the treatment field as the tumour was in a separate accessory breast which had been fully excised. The patient subsequently commenced Tamoxifen. Twelve weeks post-radiotherapy, she was in remission and all acute radiotherapy reaction had subsided.
DISCUSSION
95% of ectopic breast cancers occur in aberrant breast tissue (islands of unorganized secretory systems without any relationship to the overlying skin) compared with just 5% in a supernumerary breast (an organized ductal system which is related to the overlying skin, via a separate nipple–areolar complex) [3, 7]. They can present anywhere along the primitive milk (galactic) line, most commonly the axilla (60–70%) and infrequently on the thorax (5–10% in the inframammary region as described in our case) and rarely the vulva [1, 3]. The most common histological type is IDC, 50–79%, with 9.5% medullary and lobular carcinomas [3, 4].
Ectopic breast cancers are not detected at routine mammographic screening due to their location and may not present until signs and symptoms develop, including rapid growth of ectopic tissue, localized pain or inflammation and progressively growing nodules [1, 7].
The differential diagnosis of ectopic breast carcinoma is broad: lipoma, lymphadenopathy, follicular cysts, fibromas, tuberculosis, seborrheic keratosis and intra-dermal nevi [7]. Appropriate imaging (including both breasts) therefore needs to supplement clinical examination, with biopsy considered even if radiological findings are at variance with clinical presentation [1, 4]. MRI allows the evaluation of tumour extent and the relationship with normal breast issue prior to surgical intervention and is recommended to aid diagnosis and staging in patients presenting with a mass in the galactic line [8].
Treatment regimes in the literature follow the standardized guidelines for breast cancer [2, 3, 7, 9]. Initially, ipsilateral mastectomy of the adjacent breast was recommended; however, this provides no additional benefit and is no longer advocated [4]. Adjuvant chemotherapy and hormonal therapy is offered depending on tumour and patients characteristics.
Adjuvant radiotherapy should be considered to optimize locoregional control. A review of the literature offers no formal guidance on radiotherapy dose, fractionation and treatment fields in this setting. Treatment fields have ranged from incorporating the tumour bed alone to inclusion of the ipsilateral uninvolved breast, axilla and supraclavicular fossa, even in the absence of nodal metastases [5, 7, 8]. Our team made the decision to treat the tumour bed alone, as irradiation of the ipsilateral breast or axilla in the absence of disease was unlikely to improve local control further, and would contribute to increased toxicity, particularly cosmetic.
Electron therapy allowed the treatment of the tumour bed effectively to the desired depth, without undue toxicity, although the superior margin of the field overlapped with the inferior part of the ipsilateral breast (see Fig. 2). Doses used in the literature range from 50 to 66 Gy in 2-Gy fractions [7, 8]. We delivered a dose of 40 Gy in 15 fractions: a standardized dose for breast radiotherapy in the UK following the results of the START B trial. [10].
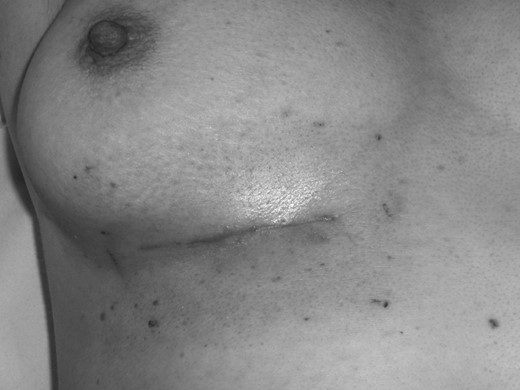
Clinical photograph illustrating the acute radiation reaction on patient's skin at completion of her electron beam therapy.
Prognosis for ectopic breast carcinoma is similar to that of an equivalent breast carcinoma [5]. However, due to its late presentation, delay in diagnosis and difficulty in screening, both lymph node and distant metastasis are more prevalent at diagnosis which impacts on prognosis [5, 10]. Additionally, due to the small size of the gland early infiltration of the skin and chest wall is a feature.
This case describes a rare diagnosis of an inframammary ectopic breast carcinoma treated with wide local excision, adjuvant radiotherapy and hormone therapy. This case highlights the importance of a high index of suspicion for investigating masses or ectopic breast tissue along the galactic line, and the importance of MRI staging prior to considering surgical and oncological options. Electron therapy can be delivered safely, reducing treatment field size and subsequent late cosmetic effects.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and case series and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.



