-
PDF
- Split View
-
Views
-
Cite
Cite
Yutaka Shimada, Tomoyuki Okumura, Shozo Hojo, Koshi Matsui, Takuya Nagata, Shinichi Hayashi, Kenichi Tazawa, Fuminori Yamagishi, Kazuhiro Tsukada, Adenocarcinoma in long-segment Barrett's esophagus 44 years after total gastrectomy, Journal of Surgical Case Reports, Volume 2013, Issue 12, December 2013, rjt100, https://doi.org/10.1093/jscr/rjt100
Close - Share Icon Share
Abstract
Although Barrett's esophagus may occur without gastric acid, Barrett's adenocarcinoma after total gastrectomy is rare. Here, we present Barrett's adenocarcinoma in long-segment Barrett's esophagus after total gastrectomy. The patient was a 74-year-old male who underwent total gastrectomy 44 years ago. An endoscopic examination revealed long-segment Barrett's esophagus starting 17 cm from the incisors and continuing 20 cm to esophagojejunostomy, with irregular mucosa 27–31 cm from the incisors. Pathological diagnosis of a biopsied specimen was adenocarcinoma. We performed subtotal esophagectomy with lymph node dissection in the prone position and reconstructed the esophagus with ileocolic interposition. Postoperative pathological diagnosis from a Barrett's epithelial section was well differentiated adenocarcinoma. This case had the longest interval from total gastrectomy and smallest oral margin of Barrett's epithelium. Our case suggested that careful surveillance is needed for patients exhibiting recurrent bile reflux following total gastrectomy.
INTRODUCTION
The incidence of esophageal adenocarcinoma is strongly associated with that of gastro-esophageal reflux disease (GERD). GERD is mainly induced by a combination of gastric acid and duodenal juice. However, bile acid and pancreatic juice without gastric acid can induce Barrett's esophagus, and a few reports have described Barrett's adenocarcinoma in patients who had undergone total gastrectomy. Here, we report a case of Barrett's adenocarcinoma in a patient who had undergone total gastrectomy 44 years ago and discuss other cases in the literature.
CASE REPORT
In October 2011, a 74-year-old male was admitted to our hospital due to ulceration of the upper to middle thoracic esophagus. The patient had undergone total gastrectomy because of ulcerative stricture of the esophagogastric junction at the age of 30. Following this surgery, he had recurrent bile acid reflux for >40 years which had increased in frequency from 10 years ago. When the patient was 62 years old, an endoscopic examination revealed long-segment Barrett's esophagus, and he subsequently underwent yearly check-up and endoscopic examinations. Irregular mucosa was found 27 cm from the incisors, and the oral margin of Barrett's esophagus was 17 cm from the incisors (Fig. 1). Frequent bile regurgitation was found during an endoscopic examination. Pathological examination of biopsied specimens from the irregular mucosa revealed adenocarcinoma.
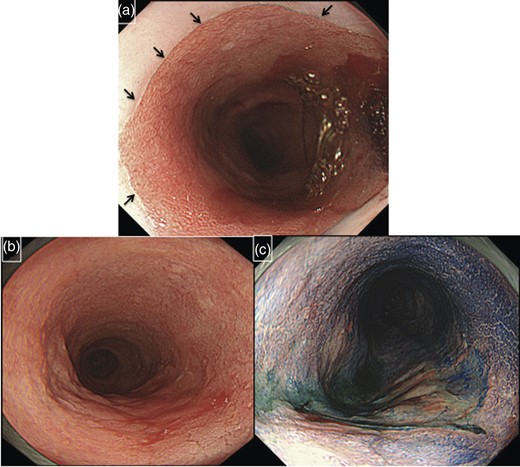
Endoscopic examination of the patient. The oral margin of Barrett's esophagus was 17 cm from the incisors (black arrows indicate the margin of Barrett's epithelium) (a). Ulceration was found 27–31 cm from the incisors (b and c). Pathological diagnosis was adenocarcinoma with Barrett's esophagus.
Although adenocarcinoma was thought to be limited to the mucosal layer, the patient requested resection for adenocarcinoma as well as Barrett's esophagus due to intractable bile reflux. We performed subtotal esophagectomy with lymph node dissection in the prone position and reconstructed the esophagus with ileocolic interposition. During laparotomy, we confirmed that jejunojejunostomy was located 10 cm from esophagojejunostomy. Postoperative pathological diagnosis from a Barrett's epithelial section was well-differentiated adenocarcinoma (T1a 2.0 × 3.0 cm) (Figs. 2 and 3). There was no lymph node metastasis (N0).
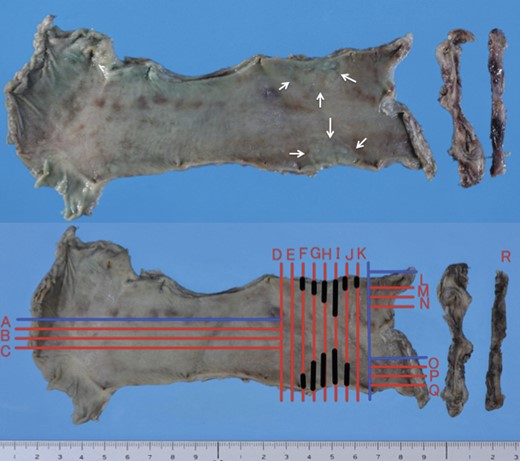
Macroscopic findings of resected esophagus and jejunum. Irregular mucosa of the upper esophagus was accidentally cut into two pieces (arrowheads) (upper side). Black lines (F, G, H, I, J and K) indicate the areas of carcinoma.
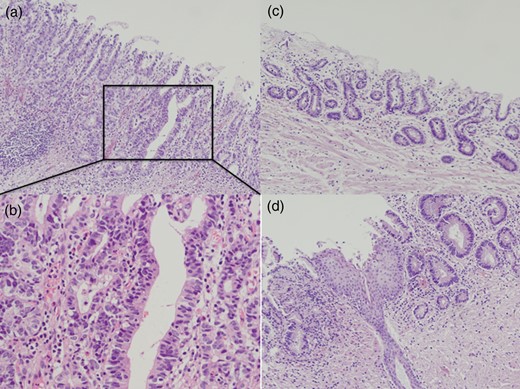
Postoperative pathological examination. A well-differentiated adenocarcinoma was revealed in Barrett's esophagus (a and b). Microsquamous epithelial islands remained in Barrett's epithelium (c and d).
Although free jejunal graft was needed due to anastomotic leakage at the site of esophagoileostomy, he was discharged 110 days after the first surgery. Final resection of the remaining Barrett's epithelium was performed concurrently with free jejunal graft. He is now well and without any evidence of recurrence.
DISCUSSION
We previously examined the effects of chenodeoxycholic acid, trypsin and hydrochloric acid individually and in different combinations on normal cultured human esophageal epithelial cells [1]. We found that cyclooxygenase-2 expression was up-regulated by bile acid, and that prostaglandin E2 production was enhanced by bile acid with trypsin, hydrochloric acid or both without a synergistic effect on cyclooxygenase-2 expression. Thus, the combination of bile acid, pancreatic juice and gastric juice was most harmful to normal esophageal epithelial cells. Miwa K et al. [2] also suggested that duodenal or gastro-duodenal contents, and not gastric contents, induced esophageal carcinogenesis through reflux. Therefore, duodenal contents may play a more crucial role in carcinogenesis than gastric acid.
Several clinical reports have also suggested that Barrett's esophagus may develop due to duodenal content reflux instead of acid reflux [3]. Recent studies suggested that Barrett's esophagus could develop 6 months after total gastrectomy [4, 5]. Of these, one had reflux symptoms and the other had no obvious symptoms. Thus, Barrett's epithelium may develop even if patients have no symptoms and a short interval from total gastrectomy. However, adenocarcinoma in Barrett's esophagus after total gastrectomy is a rare condition. Until now, only four cases have been reported in the literature [6–9] (Table 1). Including our cases, all five patients were males. Three cases had a long-term follow-up of >7 years after Barrett's epithelium. The average time between total gastrectomy and the development of Barrett's adenocarcinoma has been 34 years (ranging from 23 to 44 years). Our case had the longest interval from total gastrectomy and the smallest oral margin of Barrett's epithelium.
Characteristics of patients with Barrett's adenocarcinoma after total gastrectomy
| Case No. . | Age and Sex . | Years after total gastrectomy (years) . | Time interval between confirmation of BE and ADC (years) . | Length of Barrett's esophagus . | Location of the tumor . | Histology . | pT . | pN . | Prognosis . | Author . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64M | 39 | 17 | 10 cm | Lt | MDA | T2 | N0 | 2Y alive | Tada et al. [6] |
| 2 | 52M | 35 | 7 | >10 cm (25 cm form incisor) | Lt | WDA | T1a | N0 | 1Y alive | Konishi et al. [7] |
| 3 | 66M | 29 | unknown | 0.5 cm | Lt | MDA | T1b | N0 | unknown | Nishimaki et al. [8] |
| 4 | 69M | 23 | unknown | 13 cm | Ut, Mt, Lt | WDA, PDA, small cell carcinoma | T2 | N1 | 1.5M, died of acute vascular disease | Noguchi et al. [9] |
| 5 | 74M | 44 | 12 | 20 cm (17 cm from incisor) | Ut, Mt | WDA | T1a | N0 | 1Y10M alive | Our case |
| Case No. . | Age and Sex . | Years after total gastrectomy (years) . | Time interval between confirmation of BE and ADC (years) . | Length of Barrett's esophagus . | Location of the tumor . | Histology . | pT . | pN . | Prognosis . | Author . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64M | 39 | 17 | 10 cm | Lt | MDA | T2 | N0 | 2Y alive | Tada et al. [6] |
| 2 | 52M | 35 | 7 | >10 cm (25 cm form incisor) | Lt | WDA | T1a | N0 | 1Y alive | Konishi et al. [7] |
| 3 | 66M | 29 | unknown | 0.5 cm | Lt | MDA | T1b | N0 | unknown | Nishimaki et al. [8] |
| 4 | 69M | 23 | unknown | 13 cm | Ut, Mt, Lt | WDA, PDA, small cell carcinoma | T2 | N1 | 1.5M, died of acute vascular disease | Noguchi et al. [9] |
| 5 | 74M | 44 | 12 | 20 cm (17 cm from incisor) | Ut, Mt | WDA | T1a | N0 | 1Y10M alive | Our case |
Ut, upper thoracic esophagus; Mt, middle thoracic esophagus; Lt, lower thoracic esophagus; WDA, well-differentiated adenocarcinoma; MDA, moderately differentiated adenocarcinoma; PDA, poorly differentiated adenocarcinoma; BE, Barrett's epithelium; ADC, adenocarcinoma; T, N, TNM classification version 7.
Characteristics of patients with Barrett's adenocarcinoma after total gastrectomy
| Case No. . | Age and Sex . | Years after total gastrectomy (years) . | Time interval between confirmation of BE and ADC (years) . | Length of Barrett's esophagus . | Location of the tumor . | Histology . | pT . | pN . | Prognosis . | Author . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64M | 39 | 17 | 10 cm | Lt | MDA | T2 | N0 | 2Y alive | Tada et al. [6] |
| 2 | 52M | 35 | 7 | >10 cm (25 cm form incisor) | Lt | WDA | T1a | N0 | 1Y alive | Konishi et al. [7] |
| 3 | 66M | 29 | unknown | 0.5 cm | Lt | MDA | T1b | N0 | unknown | Nishimaki et al. [8] |
| 4 | 69M | 23 | unknown | 13 cm | Ut, Mt, Lt | WDA, PDA, small cell carcinoma | T2 | N1 | 1.5M, died of acute vascular disease | Noguchi et al. [9] |
| 5 | 74M | 44 | 12 | 20 cm (17 cm from incisor) | Ut, Mt | WDA | T1a | N0 | 1Y10M alive | Our case |
| Case No. . | Age and Sex . | Years after total gastrectomy (years) . | Time interval between confirmation of BE and ADC (years) . | Length of Barrett's esophagus . | Location of the tumor . | Histology . | pT . | pN . | Prognosis . | Author . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64M | 39 | 17 | 10 cm | Lt | MDA | T2 | N0 | 2Y alive | Tada et al. [6] |
| 2 | 52M | 35 | 7 | >10 cm (25 cm form incisor) | Lt | WDA | T1a | N0 | 1Y alive | Konishi et al. [7] |
| 3 | 66M | 29 | unknown | 0.5 cm | Lt | MDA | T1b | N0 | unknown | Nishimaki et al. [8] |
| 4 | 69M | 23 | unknown | 13 cm | Ut, Mt, Lt | WDA, PDA, small cell carcinoma | T2 | N1 | 1.5M, died of acute vascular disease | Noguchi et al. [9] |
| 5 | 74M | 44 | 12 | 20 cm (17 cm from incisor) | Ut, Mt | WDA | T1a | N0 | 1Y10M alive | Our case |
Ut, upper thoracic esophagus; Mt, middle thoracic esophagus; Lt, lower thoracic esophagus; WDA, well-differentiated adenocarcinoma; MDA, moderately differentiated adenocarcinoma; PDA, poorly differentiated adenocarcinoma; BE, Barrett's epithelium; ADC, adenocarcinoma; T, N, TNM classification version 7.
It is uncommon for columnar epithelia to reach the level of the cervical esophagus. However, a previous study described a patient with long-standing gastric acid reflux who developed adenocarcinoma in the cervical portion of a columnar cell-lined esophagus [10]. Our case also had columnar cell-lined epithelia in the cervical esophagus and may be the first case that was induced by bile reflux. Although there may be a possibility for the stasis of bile acid at the site of a natural stenosis by the aortic arch, the reason why carcinoma developed in the upper esophagus and not in lower esophagus is not clear.
The distance that is too short between esophagojejunostomy and jejunojejunostomy has already been shown to induce severe reflux esophagitis after total gastrectomy. Therefore, surgeons should not perform this procedure after total gastrectomy.
Three options were recommended as treatment strategies in our case: (i) Endoscopic mucosal resection (EMR) and follow-up, (ii) EMR plus switch operation of jejunojejunal anastomosis, (iii) resection for Barrett's adenocarcinoma as well as Barrett's esophagus. Even if patients undergo EMR, Barrett's adenocarcinoma may still develop from the remaining Barrett's epithelium. Thus, careful check-up and endoscopic examinations are needed following EMR. A switch operation for the treatment of intractable bile reflux may reduce the degree of reflux. However, Barrett's epithelium does not disappear after this procedure. In our case, the patient strongly refused to undergo a follow-up endoscopic examination and requested resection for Barrett's adenocarcinoma as well as Barrett's esophagus.
In conclusion, our case suggested that chronic esophagitis by the reflux of duodenal contents induced Barrett's adenocarcinoma; therefore, careful long-term surveillance is necessary for symptomatic patients who have undergone total gastrectomy. Surgeons should not perform procedures that may lead to bile reflux to the esophagus.
Acknowledgements
We thank Mr. David A Coolidge for editing the manuscript.



