-
PDF
- Split View
-
Views
-
Cite
Cite
M Ahmad, J Vatish, A Willis, R Jones, R Melhado, Embolisation of an acute inflammatory pulmonary artery aneurysm using an Amplatzer® vascular plug, Journal of Surgical Case Reports, Volume 2012, Issue 8, August 2012, Page 15, https://doi.org/10.1093/jscr/2012.8.15
Close - Share Icon Share
Abstract
Pulmonary artery aneurysms (PAA) are rare. To date there are no cases in the literature describing formation secondary to oesophageal perforation. We present an unusual case of ruptured inflammatory segmental PAA.
A patient with oesophageal squamous cell cancer presented with shortness of breath and sepsis following endoscopic dilatation of an oesophageal stricture. Imaging demonstrated oesophageal perforation and a pulmonary parenchymal collection containing an inflammatory PAA. Following initial conservative management, he then re-presented with haemoptysis secondary to PAA rupture. He was treated with embolisation using an Amplatzer® vascular plug (AVP) and went on to make an uneventful recovery.
INTRODUCTION
Inflammatory pulmonary artery aneurysms (PAA) are uncommon complications of pulmonary inflammation and sepsis (1). Aetiology includes acute or chronic pulmonary infection or inflammation and congenital abnormalities. Rarer causes include trauma, necrotic neoplasms and iatrogenic injury(2,3). Symptoms range from sepsis due to the underlying lung infection, pain or life-threatening haemoptysis (4).
The Amplatzer® vascular plug (AVP; AGA Medical, Golden Valley, MN, USA) is a relatively new device used in transcatheter embolisations. To date its application within the pulmonary vessels has been predominantly described in the treatment of arteriovenous malformations (5).
We describe a patient with an inflammatory PAA, secondary to oesophageal perforation, successfully treated with an AVP.
CASE REPORT
A 62 year old male presented to our unit with dysphagia 17-months after being diagnosed with metastatic oesophageal squamous cell carcinoma (SCC). He had previously undergone palliative chemo-radiotherapy for symptom control.
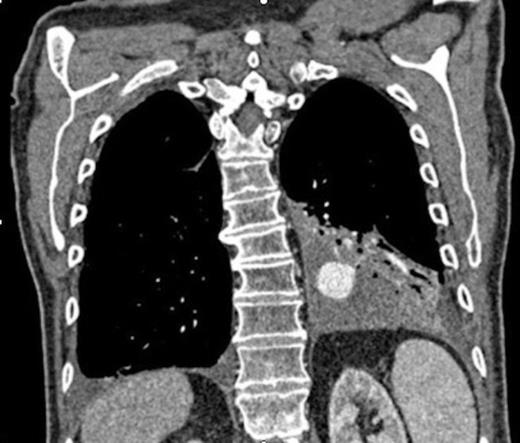
Coronal CT image in arterial phase demonstrating a 28mm diameter left lower lobe pulmonary artery aneurysm in association with a fluid collection
Upper gastrointestinal endoscopy demonstrated a post-radiotherapy oesophageal stricture at 35cm. CT showed progression of lung metastases and new liver metastases. We performed dilatation to 11 FR (French) using Savary-Gillard dilators, during which a mucosal tear was noted, but a post-operative contrast swallow did not demonstrate a perforation and he was discharged home.
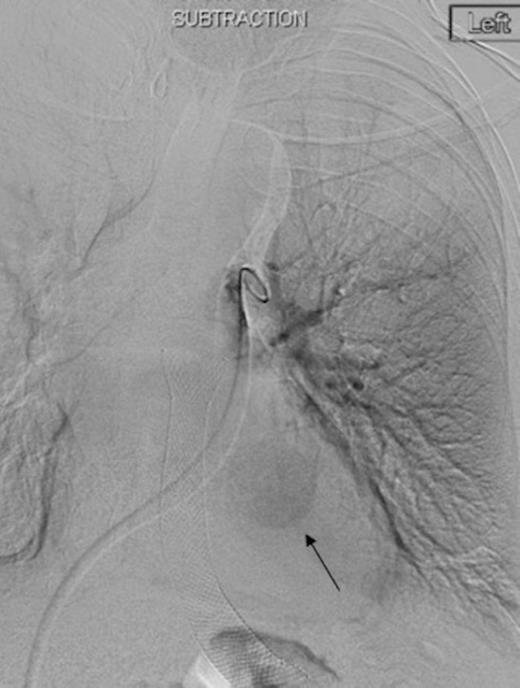
Left pulmonary artery digital subtraction angiography image demonstrating filling of left lower lobe aneurysm (arrow)
Two weeks later he re-presented with fever and shortness of breath. CT-thorax demonstrated a left lower lobe cavity containing a 14mm segmental PAA. Contrast-swallow confirmed oesophageal perforation at the site of the oesophageal stricture in continuity with this cavity. This was drained percutaneously under radiological guidance and a partially-covered MicroTech® stent was inserted to control the oesophageal leak. Culture of the abscess cavity pus grew Candida albicans which was treated with appropriate antimicrobials. The pulmonary cavity reduced in size on serial CTs and he was discharged home.
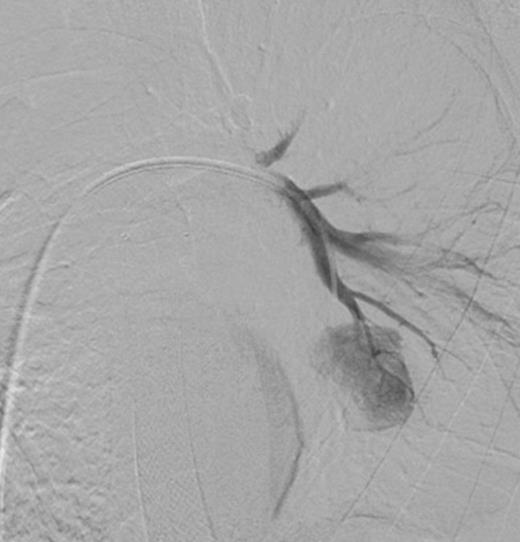
Selective digital subtraction angiography image of left lower lobe pulmonary artery, demonstrating filling of the inflammatory aneurysm and depicting the feeding branches clearly
Three days later he presented with significant haemoptysis. A CT pulmonary angiogram (CTPA) demonstrated an increase in size of the PAA from 14mm to 28mm (Figure 1) diameter. A radiology opinion was sought and the patient transferred to the angio-suite for intervention.
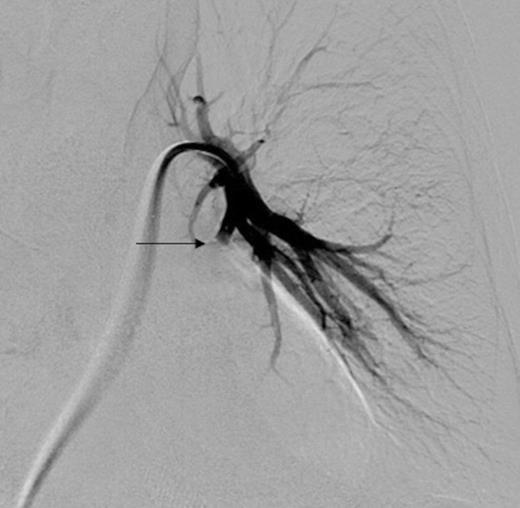
Digital subtraction angiography post deployment of the AVP 4 device in the PA branch feeding the aneurysm (arrow). No residual or collateral filling seen
Using the right femoral vein for access, a 5 FR (French) pigtail catheter was advanced into the main left pulmonary artery. Angiography demonstrated the vascular anatomy in relation to the PAA (Figure 2). The pigtail catheter was then exchanged for a 7 FR Destination access sheath (Terumo, Japan). A 5 FR multipurpose catheter and hydrophilic guidewire combination was used to selectively catheterise the segmental left lower pulmonary arterial branch feeding the aneurysm (Figure 3). This was followed by deployment of a single 7mm Amplatzer® Vascular Plug 4 (AVP 4) in the feeding branch, with preservation of the surrounding pulmonary artery branches (Figure 4). A repeat CTPA was undertaken 2 days later, confirming satisfactory PAA exclusion, with no evidence of residual filling (Figure 5). The patient made an uncomplicated recovery and was discharged home, but unfortunately died 6-months later from metastatic disease.
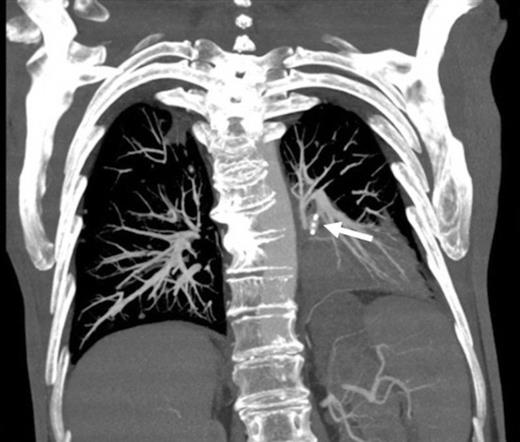
Coronal reconstruction of CTPA taken 2 days after embolisation demonstrating successful ongoing exclusion of the PA aneurysm. Note the AVP (arrow) and continued filling of unaffected lower lobe segmental pulmonary arterial branches
DISCUSSION
PAA are rare, constituting less than 1% of all thoracic aneurysms (1). Congenital abnormalities associated with pulmonary hypertension are responsible for 50% of all PAA (6). Other causes include connective tissue diseases, vasculitides, trauma, iatrogenic injury and infection (2).
PAA can be relatively asymptomatic until the point of rupture. A high index of suspicion is required because clinical signs may be non-specific and attributable to other causes. Patients may complain of chest pain, cough, haemoptysis or dyspnoea from external compression onto the bronchi. The usual presentation at time of rupture is massive haemoptysis and haemodynamic instability. Most PAA are visible on plain x-ray, but may be misidentified and attributed to other causes such as pulmonary nodules, hilar adenopathy or metastases (2). CT imaging can be used to diagnose PAA and is the initial investigation of choice followed by percutaneous pulmonary arteriography. The classic appearance of PAA is of rapid filling with delayed emptying (1).
Inflammatory PAA, in contrast to other causes of aneurysm, tend to grow rapidly with an increased risk of rupture. Left untreated, ruptured PAA are invariably fatal. Previously, treatment options were limited to surgical resection including pneumonectomy in the case of proximal pulmonary artery lesions, lobectomy in peripheral cases, or resection with segmental prosthetic graft repair (7). Post-operative mortality however is high, with the literature suggesting rates ranging from 35-100%, and often patients with PAA are not fit for surgery (1). There is some evidence to suggest alternative conservative treatment, applicable only in non-ruptured PAA, such as intravenous antimicrobials can be used to treat selected cases (7).
Advancements in endovascular therapies have resulted in significant improvements in the management of PAA. Techniques include detachable coil embolisation, with the literature reporting impressive initial success rates as high as 95%, or the use of gelfoam, balloons or vascular plugs (1,5,8).
The Amplatzer® 4 vascular plug is a relatively new occlusion device, used for transcatheter embolisation and deliverable through a conventional 0.038 inch catheter lumen. It is a self-expanding cylindrical mesh device made of nitinol wires that are compressed whilst loaded in its delivery catheter, but on being deployed, expand and adapt to occlude the target site (9). Another advantage is that it can be safely positioned and re-positioned if necessary within target vessels prior to deployment. The clinical use of AVP 4 has predominantly been in the embolisation of pulmonary arteriovenous malformations and in the peripheral vasculature for treatment of aortoiliac aneurysms where it is used on its own or in conjunction with other techniques such as embolisation coils (5,9). To date, we have found one report in the literature where it has been used in conjunction with micro-coils to successfully treat PAA in a case of Hughes-Stovin syndrome (10) and a further report of a paediatric case where AVP was used on its own to treat an idiopathic PAA (8).
Our case illustrates the successful endovascular treatment of an inflammatory PAA, which is an extremely rare and potentially catastrophic complication of oesophageal perforation. Specifically, an AVP 4 device was used in isolation and to our knowledge this is the first report of its use in this setting.



