-
PDF
- Split View
-
Views
-
Cite
Cite
X Ye, S Warrier, K J Nejad, A D Parasyn, Subcutaneous spreading squamous cell carcinoma in a patient with epidermolysis bullosa, Journal of Surgical Case Reports, Volume 2012, Issue 8, August 2012, Page 10, https://doi.org/10.1093/jscr/2012.8.10
Close - Share Icon Share
Abstract
Epidermolysis Bullosa (EB) is a complex group of genetic disorders characterised by mechanical fragility in the basement membrane zone. Affected individuals experience significant morbidity and mortality, most commonly from cutaneous malignancies. In fact, 90.1% of EB patients develop Squamous Cell Carcinoma (SCC) before the age of 55, 80% of whom die within 5 years of diagnosis. Furthermore, the management of cutaneous malignancies in EB is fraught with challenges given the atypical presentations of malignancies and the co-existence of systemic co-morbidities. To illustrate the common pearls and pitfalls of managing EB in the perioperative setting, we present a case of SCC of the left forefoot which spread through a natural plane of weakness in the dermal-epidermal junction as a complication of the congenital weakness in the area resulting from EB.
INTRODUCTION
Epidermolysis Bullosa (EB) is a complex group of genetic disorders characterised by mechanical fragility in the basement membrane zone (1). Although rare, affected individuals experience significant morbidity and excessive mortality, most commonly from cutaneous malignancies (1). In fact, 90.1% of EB patients develop Squamous Cell Carcinoma (SCC) before the age of 55, 80% of whom die within 5 years of diagnosis (2). The explanation for this is multifactorial. First, carcinogenesis is elevated in EB patients at a molecular level and as a result of chronic trauma, most commonly in the limbs (3). Second, cutaneous malignancies tend to occur in multiples and in occult, non-sun exposed sites. Third, the clinical behaviour of SCCs in EB is disproportionately aggressive compared to the histological grade and even the most well differentiated lesions convey a poor prognosis (3).
We present below a case of a 37 year old man with SCC of the left forefoot, which spread through a natural plane of weakness in the dermal-epidermal junction as a complication of the congenital weakness in the area resulting from EB. To the best of our knowledge, this is the first case report to document the highly unusual pattern of occult spread of SCC.
CASE REPORT
A 37 year old man with EB presented to the Surgical Oncology unit with a 7.0 x 3.5cm Marjolin’s ulcer on the sole of his left foot, covering the area from the first metatarsal-phalangeal joint to the great toe (Fig. 1). The patient was otherwise well but reports a history of chronic ulceration and delayed healing in the affected foot stemming from minor trauma to the area.
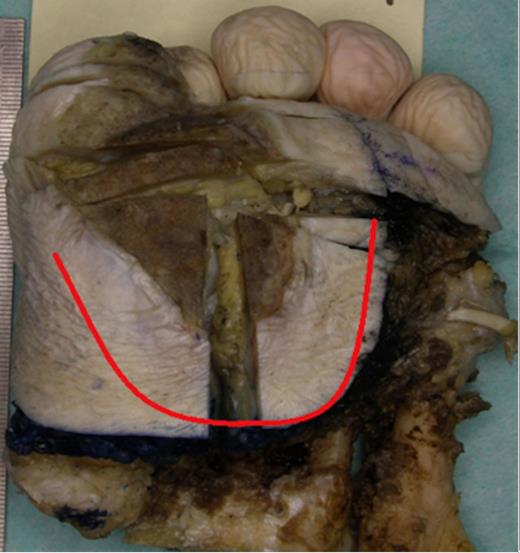
Plantar view of the left forefoot showing a large keratinising Squamous Cell Carcinoma over the medial aspect of the forefoot extending to the plantar aspect of the great toe, accompanied by extensive superficial invasion of the subcutis, extending 15mm radially from area of ulceration (red line)
There was no loco-regional lymphadenopathy. However, palpation of the surrounding skin revealed a significant area of induration beyond the edges of the ulcer (Fig. 1). A magnetic resonance image (MRI) of the foot demonstrated an ill-defined mass spreading along the subcutaneous fat from the superficial margin of the plantar plate of the first metatarsal-phalangeal joint to the proximal margin of the forefoot (Fig. 2). In context of the clinical presentation, the patient was diagnosed with infiltrative SCC and a left forefoot amputation was performed.
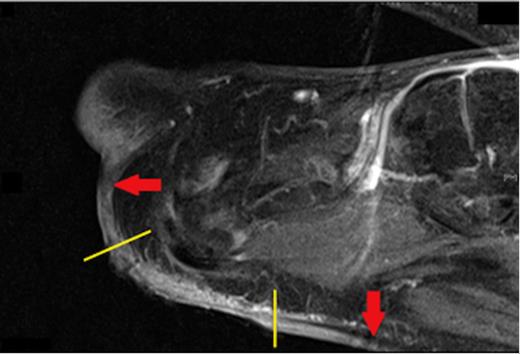
Sagittal view of a T1 weighted MRI showing the extent of subcutaneous spread of the Squamous Cell Carcinoma (red arrows) beyond the visible edges of the ulcer (yellow lines)
Pathological examination of the plantar fascia revealed extensive invasion of the subcutis, extending radially for up to 15mm beyond the area of ulceration (Fig. 1). The tumour was 6mm thick and confined to the subcutaneous plane, well clear of the underlying muscle and bone. Microscopic examination of indurated areas revealed a moderately differentiated SCC invading the deep dermis in a band-like fashion parallel to the epidermis without evidence of peri-neural or deep structure involvement (Fig. 3).
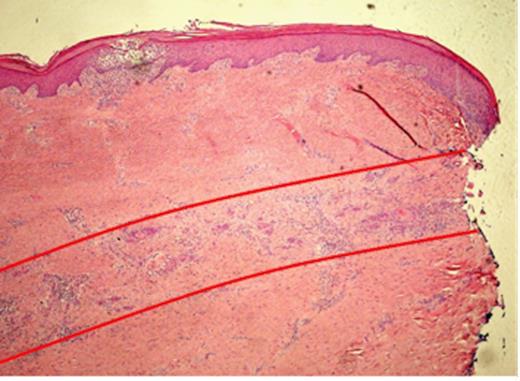
Histopathological slide of the indurated skin taken 10mm away from the ulcer showing a moderately differentiated Squamous Cell Carcinoma invading the deep dermis in a band like fashion, parallel to the epidermis (red lines). The local infiltration is well confined to the subcutaneous plane without peri-neural or deep structure involvement
The patient’s postoperative course was complicated by delayed wound healing and cellulitis at the surgical site.
DISCUSSION
The clinical management of cutaneous malignancies in EB is fraught with challenges given the aggressive nature of SCCs and the co-existence of systemic co-morbidities (1,3).
As demonstrated in the present case, the evaluation of SCC in EB requires MRI to define the true extent of the lesion (2). Further, amputation has to date been the most preferred method of ‘treatment’ for plantar SCCs with soft tissue involvement, as the efficacy of Moh’s surgery and sentinel lymph node biopsy remain inconclusive (2). Whilst neo/adjuvant therapy chemotherapy and radiotherapy may be used, there is a lack of data supporting their impact on prognosis (2).
Anaesthetic consultation should be sought early. Patients with EB may have protein malnutrition from loss of blood and serum in cutaneous and mucosal wounds as well as gastrointestinal complications such as oesophageal strictures (3).
Endotracheal intubation may be complicated by ankyloglossia, poor dentition, buccal fragility and limited mouth opening (4). The risk of mucosal injury also exists. As such, lubricated facemask and regional anaesthesia have been recommended in compliant patients (5). Also, proper positioning and ample padding is essential to prevent skin and soft tissue damage, especially as many patients have co-existing joint contractures. Adequate warming and careful transfers should also be employed for the same reason (5).
Meticulous wound care and immobilisation are critical as EB patients are predisposed to delayed wound healing (from a deficiency of Laminin-5, a keratinocyte adhesion factor) and surgical site infections (SSIs) (from accumulation of serum in the dermal-epidermal junction and impaired lymphocyte function) (3,5,6). Interestingly, the SSIs in our case were also dermal in origin and uncharacteristically aggressive, requiring 2 months of antibiotics, silver dressings and immobilisation.
Numerous molecular therapies are currently being trialled with promising results (1). These include gene therapies (via such in vivo methods as the gene gun and ex vivo methods involving autografting patients with cultured sheets of genetically modified keratinocytes), protein therapies using intradermal injection of deficient proteins, autotransplantation of marrow-derived mesenchymal stem cells, and enhanced wound healing with thymosin-β4 (1,3). Low dose isoretinoin is a well-tolerated method of chemoprevention although further research is needed to explore its efficacy (2,3).
EB is a rare disorder which can result in significant morbidity and mortality. Fortunately, the perioperative management of cutaneous malignancies in patients with EB can be streamlined through better understanding of the pathophysiological basis of EB. This allows the astute clinician to pre-empt the common complications encountered in the surgical setting and institute appropriate measures in an efficient and timely manner.



