-
PDF
- Split View
-
Views
-
Cite
Cite
A Daunton, S Puig, P Taniere, C Forde, D Alderson, ON Tucker, Prevention is better than cure, Journal of Surgical Case Reports, Volume 2012, Issue 6, June 2012, Page 14, https://doi.org/10.1093/jscr/2012.6.14
Close - Share Icon Share
Abstract
The vast majority of gastric cancers are sporadic. However, 1-3% arise as a result of inherited gastric cancer predisposition syndromes, generally referred to as hereditary diffuse gastric cancer (HDGC). Of those families that fulfill the clinical criteria for HGDC only 25% have a CDH1 germline mutation. No reliable surveillance technique exists for individuals with HDGC. Difficult decisions have therefore to be made by mutation carriers to proceed with prophylactic total gastrectomy, or undergo lifelong annual surveillance. We present a case of the management of a patient with a documented CDH1 mutation and briefly review the available literature.
INTRODUCTION
Gastric cancer is the seventh most common cause of cancer death in the UK, and the second most common worldwide (1,2). In the UK, the majority of patients present with advanced stage disease with an overall 5-year survival of 15% (1). Only 20% of patients undergo radical resection in an attempt at cure. Following resection, 5-year survival rates vary from 20-30% in patients with locally advanced disease to 70-95% with disease confined to the mucosa and submucosa (3).
Whilst the majority of gastric cancer cases are sporadic, approximately 10% are familial, and 1-3% are due to recognized familial predisposition syndromes with an autosomal dominant inheritance pattern (4,5). Inherited gastric cancers are more commonly of the diffuse type or linitis plastica and are generally referred to as hereditary diffuse gastric cancer (HDGC) (5,8). These cancers are associated with a poor survival, as they tend to present late with incurable disease. Updated consensus guidelines for clinical management of HDGC were reported in 2010 (5).
CASE REPORT
A 43-year-old asymptomatic lady was referred to a clinical geneticist for genetic counseling and screening following the deaths of her 43 year-old brother and 19 year-old nephew from gastric cancer. Following confirmation of a germline mutation of the CDH1 (E-cadherin) gene she was referred to our service for discussion regarding a prophylactic total gastrectomy. She underwent a gastroscopy and CT scan of abdomen and thorax, which were both unremarkable. Six circumferential random biopsies were taken from the antrum, transitional zone, body, fundus, and cardia of the stomach, totalling 30 biopsies. They demonstrated mild chronic inflammation only following specialist histopatholical evaluation. All investigations were reviewed and discussed in detail at the Upper GI multidisciplinary team meeting (MDM).
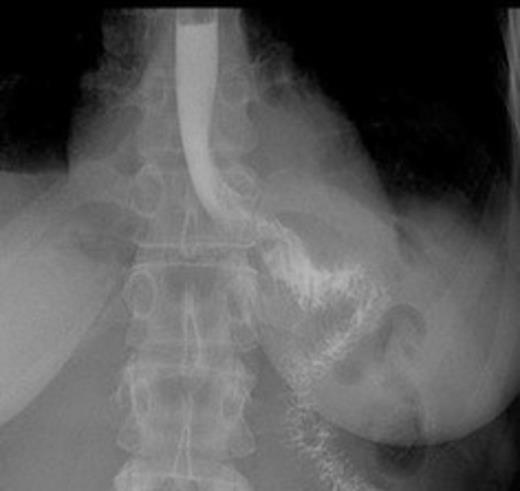
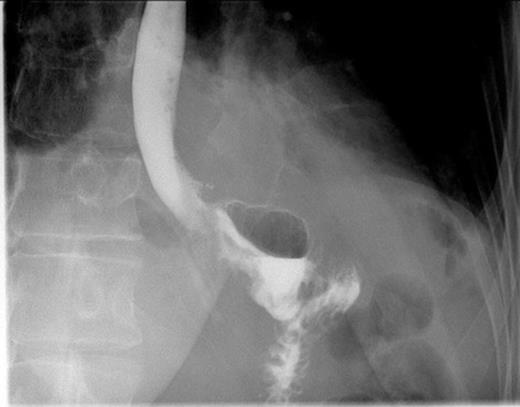
Postoperative contrast swallow
Following detailed discussion with the patient and her husband, she elected to undergo a prophylactic total gastrectomy. A laparoscopic total gastrectomy with limited D2 lymphadenectomy, Roux-en-Y reconstruction, and feeding jejunostomy insertion was performed. The stomach was removed through an extension of a left-upper-quadrant port site. The integrity of the oesophago-jejunal anastomosis was assessed by intraoperative endoscopy and a leak test.
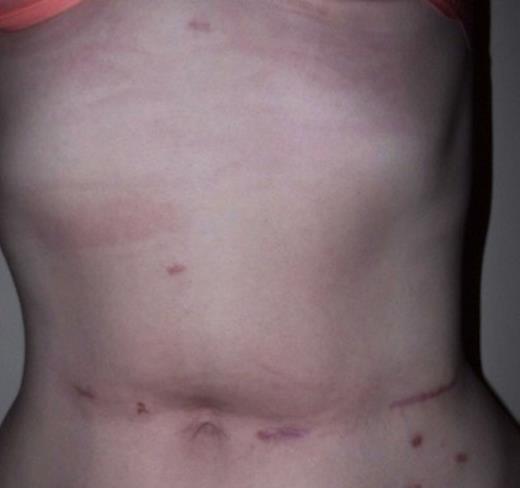
The whole stomach wall was submitted for histological microscopic examination in 67 blocks. No evidence of dysplasia or early foci of signet ring cell carcinoma were seen. The resected lymph nodes were unremarkable. The patient’s postoperative course was uncomplicated with return of bowel function on day 6 and discharge home on day 11. A postoperative contrast swallow was normal on postoperative day 3 (Figure 1). She remains well 5 months following surgery, with satisfactory wound healing (Figure 2).
DISCUSSION
This case illustrates the difficulties encountered by HDGC family members, including genetic testing, the role of surveillance versus prophylactic total gastrectomy, and the optimal surgical approach.
Genetic testing is recommended in all HDGC family members. 25-30% of families fulfilling the criteria for HDGC have a germline mutation of the CDH-1 gene.5 CDH-1 codes for the cell-cell adhesion molecule E-cadherin, which is essential for proper intercellular adhesion within epithelial tissues (8). E-cadherin appears to function as a tumour suppressor, whose loss has been documented widely in both sporadic and familial gastric cancer. Published data suggest that the penetrance of CDH-1 gene mutations is high with an estimated risk of 80% lifetime risk of gastric by age 80 and 60% lifetime risk of lobular breast cancer by age 80 (5). The causal germline mutations in HDGC cases without an identified CDH-1 mutation are currently unknown.
Despite its clear aetiology, management of HDGC family members is difficult. Gastroscopic surveillance and random biopsies are unreliable as submucosal disease is common (4). Other surveillance techniques remain experimental (6). For these reasons, prophylactic gastrectomy is recommended in confirmed CDH-1 mutation positive patients with normal gastric biopsies, once the patient is older than 20 years of age (9). Gastrectomy is a major procedure with a reported in-hospital mortality of 2-3%, and approximately 10% risk of significant complications (10). Although the risk of death in CDH-1 mutation carriers over the age of 20 years due to gastric cancer exceeds the mortality risk following prophylactic total gastrectomy, consideration of the individual patient’s wishes, and their physical and psychological fitness is paramount following a detailed discussion on the risks and benefits of surgery (6). A multidisciplinary approach to inform and counsel each patient prior to surgery is therefore essential. Patients should be managed in a high volume centre with low perioperative mortality and morbidity rates.
The standard operative approach in most centres involves a laparotomy and total gastrectomy with Roux-en-Y reconstruction with a minimum of a 50 cm roux limb to reduce the risk of biliary reflux. The proximal resection margin must traverse the distal oesophagus to ensure complete excision of all gastric cardia mucosa. A radical lymphadenectomy is not required due to the low risk of lymph node metastasis in gastric adenocarcinoma confined to the mucosa. In our patient a limited D2 lymphadenectomy was performed.
Minimally invasive approaches to distal and total gastrectomy have been developed to reduce surgical trauma and optimize patient recovery. Laparoscopic total gastrectomy would appear to be an ideal approach to minimize operative trauma and morbidity in patients undergoing a prophylactic resection. Laparoscopic gastric resection is reported to be associated with reduced perioperative morbidity and mortality, including earlier return of bowel function, oral intake, and discharge from hospital (7). However, there is limited experience and expertise in Europe with this approach. Worldwide, only 96 prophylactic gastrectomies for confirmed CDH-1 mutation have been reported which include only a small number of laparoscopic and laparoscopic-assisted approaches, the first of which was reported in 2007 (4,5) Laparoscopic total gastrectomy should only be performed in specialist centres with expertise in the technique and prospectively collected audited data to confirm the absence of additional risk.
Further research is required to identify the causative mutations in the 70-75% of HDGC family members without a CDH-1 mutation, in addition to development of more reliable surveillance approaches to prevent unnecessary prophylactic gastric resection and to inform joint decision making.