-
PDF
- Split View
-
Views
-
Cite
Cite
T Kuwata, T Iwata, T Iwanami, Post-thymectomy myasthenia gravis with an episode of Osserman stage III, Journal of Surgical Case Reports, Volume 2012, Issue 5, May 2012, Page 3, https://doi.org/10.1093/jscr/2012.5.3
Close - Share Icon Share
Abstract
Here, we present the first case of post-thymectomy Myasthenia Gravis with onset at Osserman stage III. An 81-year-old woman was admitted for an abnormal shadow seen in a chest radiograph. She had no symptoms of Myasthenia Gravis. Acetylcholine receptor antibody was within the normal range. Chest computed tomography (CT) showed a bulky anterior mediastinal tumor. She was diagnosed as having thymoma by tissue biopsy under CT guidance. The tumor was completely resected by performing thymothymectomy, left upper lobectomy, pericardial resection, and phrenicectomy. Pathological examination of the tumor identified it as a thymoma (type B2, Masaoka stage II). Two months after the surgery, she experienced the onset of post-thymectomy myasthenia gravis with Osserman stage III. The acetylcholine receptor antibody level was remarkably elevated (220 nmol/L); however, there was no evidence of tumor recurrence.
INTRODUCTION
Thymectomy is generally recommended for patients with thymoma. However, Myasthenia Gravis occasionally develops postoperatively in patients who have had thymoma despite no signs of Myasthenia Gravis before the surgery. Some studies (1-8) have reported post-thymectomy Myasthenia Gravis (PTMG); however, its mechanism and risk factors remain unclear. Moreover, no case of PTMG with onset at Osserman stage III has been reported. Here, we present the first case of PTMG at Osserman stage III.
CASE REPORT
An 81-year-old woman was admitted to our hospital for examination and treatment of an abnormal shadow seen on chest radiograph. She had no symptoms on admission. Tumor markers were within the normal range. Acetylcholine receptor antibody (ARA) was negative. Her lung function was normal. Chest computed tomography (CT) showed a bulky mediastinal tumor bulging outward into the left thoracic space (Figure 1).
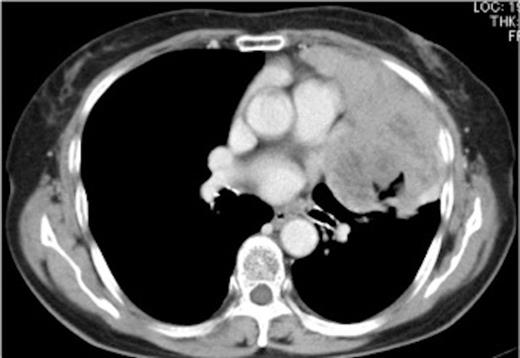
Preoperative chest computed tomography (CT) showing a bulky mediastinal tumor bulging outward into the left thoracic space and invading the pericardium and pleura.
The tumor was diagnosed as a thymoma by tissue biopsy under CT guidance. The operative findings of the tumor was completely resected by performing thymothymectomy, left upper lobectomy, pericardial resection, and phrenicectomy with median sternotomy and superior longitudinal anterior mediastinotomy. The most part of tumor had a place in the left pleural space and invaded the left superior lobe of the lung, pericardia and left phrenic nerve. Pathological examination of the tumor identified as a thymoma (Masaoka stage II). She was discharged 40 days after the surgery without postoperative complications. Two months after the surgery, the patient was admitted to our hospital for respiratory failure requiring mechanical ventilation. ARA level was remarkably elevated (220 nmol/L). Evoked electromyography showed waning. However, there was no evidence of tumor recurrence on imaging examination. We diagnosed the patient as having PTMG with Osserman stage III and started steroid pulse therapy (prednisolone 1g/day for 3 days). Additionally, we treated the patient with immunoadsorption to shorten the duration of the steroid therapy. At three months after the admission, she was removed from the ventilator with a drug regimen of 60 mg/day prednisolone. Moreover, the ARA level reduced to 43.5 nmol/L (Figure 2).
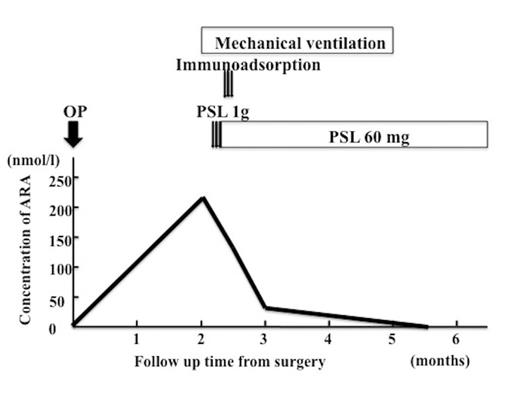
DISCUSSION
Here, we present the first case of myasthenia gravis onset with Osserman stage III after thymectomy. Studies have reported that the incidence of PTMG is 0.9–20% (1-6).However, we could not find reports of PTMG occurring at Osserman stage III.
The current hypothesis of the pathogenic mechanism of PTMG includes: [1]thymoma recurrence (7,8); [2] surgical exposure to larval MG (2); and [3]activation of peripheral lymphocytes from thymoma after surgery (9,10). The risks of developing PTMG have been documented in various studies. However, these studies did not highlight risks such as operative method that may be statistically significant. In this regard, Nakajima et al reported that all patients with Myasthenia Gravis before or after thymectomy had high ARA levels at the onset of Myasthenia Gravis. Thus, high ARA level might be a predictive indicator of PTMG (6).
In our case, postoperative imaging examination and preoperative physical findings showed that PTMG did not develop because of the factors highlighted in hypotheses 1 and 2. PTMG onset was more likely to be caused by the factors described in hypothesis 3. The methods for evaluating the hypothesis 3 are currently available for clinical use. However, we regret that we evaluated ARA at an earlier date after the surgery. Moreover, after the surgery we might examine her about Myasthenia Gravis such as Harvey-Masland test.
We experienced a case of PTMG onset at Osserman stage III with a negative preoperative ARA. We conclude that surgery of thymoma needs close and detailed pre- and postoperative myasthenia gravis examinations to screen for PTMG onset. We believe that this case may offer some useful information for patients with thymoma, be considered surgery.



