-
PDF
- Split View
-
Views
-
Cite
Cite
Vanessa Chow, Kate Gardner, David Howlett, Primary localized laryngeal amyloidosis presenting with dysphonia: a case report, Journal of Surgical Case Reports, Volume 2012, Issue 11, November 2012, rjs005, https://doi.org/10.1093/jscr/rjs005
Close - Share Icon Share
Abstract
Localized laryngeal amyloidosis is a rare disease with poorly understood aetiology. The commonest symptom at presentation is dysphonia and for a correct diagnosis of amyloidosis to be made a high index of suspicion is needed [Fraihat A, Ardah A. Laryngeal amyloidosis: a case report. J R Med Serv 2005;17:57–9; Passerotti GH, Caniello M, Hachiya A, Santoro PP, Imamura R, Tsuji DH. Multiple sited amyloidosis in the upper aerodigestive tract: case report and literature review. Rev Bras Otorrinolaringol 2008;74:462–6]. We present a case of a 48-year-old male who was investigated over a 5-year period for persistent and progressive hoarseness of voice before the accurate diagnosis of localized amyloidosis was reached. Management of this case consisted of local treatment with endoscopic carbon dioxide laser excision of laryngeal lesions to good effect and exclusion of systemic disease with yearly follow-up for monitoring disease progression.
INTRODUCTION
Laryngeal amyloidosis has been estimated to account for 0.2–1.2% of all benign laryngeal tumours. The symptoms are largely dependent on the location and size of the deposits. As illustrated in the case study, typically the only manifest symptom is persistent and progressive dysphonia. This common symptom makes the diagnosis of amyloidosis difficult and highlights the need for a high index of clinical suspicion from ENT surgeons, histopathologists and radiologists for a timely diagnosis.
CASE STUDY
A 48-year-old male presented repeatedly to otolaryngology with progressive hoarseness of voice over a 7-year period. He had no symptoms of dyspnoea, dysphagia or stridor. He was otherwise well and did not smoke or drink alcohol.
He initially presented after 18 months with hoarseness of voice. Laryngoscopy revealed oedematous and thickened vocal cords bilaterally. There were no other significant findings on clinical examination. The patient was diagnosed with chronic laryngitis and treated with antibiotics and speech therapy.
He re-presented after 12 months with progression of symptom of hoarseness. Repeated laryngoscopy demonstrated oedematous vocal cords with prominent false vocal cords. He was treated for gastro-oesophageal reflux disease.
Four years later he re-presented with the same symptom. An endoscopy revealed oedematous vocal cords and a hard prominent left supraglottic/false vocal cord polypodial mass. This was treated using a carbon dioxide (CO2) laser to remove the lesion.
Histopathology revealed prominent acellular, eosinophilic deposits within the stroma (Figs 1 and 2). Under polarized light, Congo red staining showed the characteristic apple-green birefringence of amyloidosis. No dysplasia or malignancy was seen.
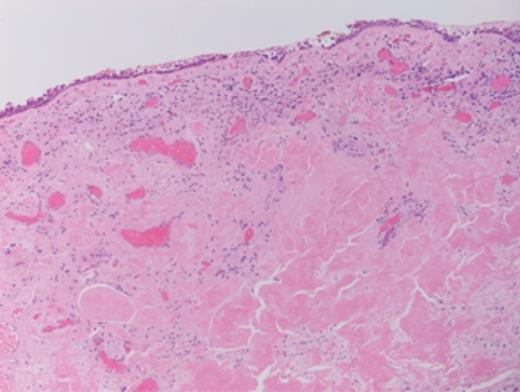
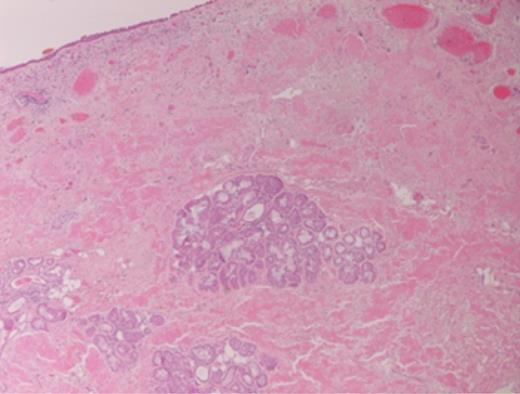
Further investigations demonstrated normal full blood count, erythrocyte sedimentation rate, serum calcium, renal and liver function tests, immunoglobulin levels. Serum protein electrophoresis did not reveal the presence of a paraprotein. No serum free light chains were detected. Bone marrow aspiration and trephine showed a normal marrow, with no infiltration. A computerized tomography scan of the chest, abdomen and pelvis did not demonstrate any signs of systemic disease.
Post treatment with CO2 laser, the patient underwent a T1 weighted magnetic resonance imaging (MRI) with gadolinium of the neck. The imaging demonstrated an abnormal soft tissue density in the left supraglottic region (Figs 3 and 4). The low intensity lesion was 9 × 4 mm in size and caused distortion of the left aryepiglottic fold and minor airway narrowing. The findings on MRI suggested the persistence of disease.
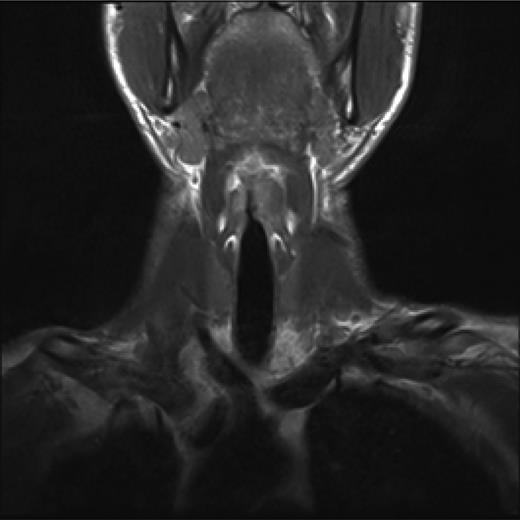
Coronal view, T1 weighted MRI neck showing abnormal low intensity foci in the left false vocal cord.
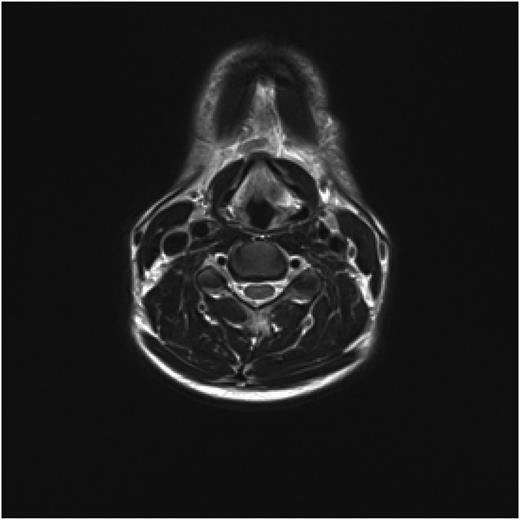
Axial view of MRI neck demonstrating soft tissue abnormality in the left supraglottic area.
DISCUSSION
Amyloidosis is an uncommon, progressive disease characterized by extracellular deposition of insoluble fibrillar proteins in tissues leading to organ failure. It can be classified into systemic disease or localized to a single organ (10–20% cases are localized) [1,2].
Systemic amyloidosis can be further divided either by precursor proteins (of which 25 precursor proteins have been currently identified) or by the older classification into hereditary amyloidosis (including familial Mediterranean fever (AA, AF)), primary (idiopathic) systemic amyloidosis (AL) and secondary (reactive) systemic amyloidosis (AA) as a reaction to an underlying inflammatory condition. Different subtypes have very different natural histories and different therapies are used [2,3].
In localized amyloidosis, amyloid protein deposition is limited to a single organ. Two theories account for the amyloid deposition; first, the local synthesis of amyloid within the target organ produces the proteins locally resulting in only single organ involvement. Second, the distant or systemic light chains are producing amyloid which deposit in the target organ due to a localized pathological process such as inflammation [3–5].
The diagnosis of amyloidosis requires tissue biopsy, with Congo red immunohistochemical staining showing a pink or red colour under normal light and the classical apple-green birefringence under polarized light (Fig. 5). Once localized amyloidosis has been established, it is important to diagnose or exclude systemic involvement. The natural history of systemic amyloidosis is to progress much more quickly accounting for a prognosis of <2 years from diagnosis in patients with AL disease. This contrasts with an excellent prognosis in localized amyloidosis [3,5].

Congo red staining of the biopsy sample. Histology shows an acellular, amorphous, eosinophilic material deposit in the stroma, with perivascular and periglandular accentuation. Typing of the specimen is based on clinical and laboratory findings (including paraproteinaemia looking for an underlying myeloma, TTR gene rearrangement studies looking for the genetic defect in familial amyloid.)
There is no consensus on how extensively patients diagnosed with amyloidosis should be investigated for systemic disease. In the case above, the patient underwent radiological, biochemical and bone marrow biopsy to exclude evidence of systemic amyloidosis. This comprehensive series of investigations is not always seen to be necessary and Lewis et al. study on localized laryngeal amyloidosis at the Mayo clinic recommended urine and serum electrophoresis only, with invasive investigations such as bone marrow and bowel biopsies deemed unnecessary as the vast majority of cases of laryngeal amyloidosis are localized [6].
The role of radiological investigations is mainly supportive of the diagnosis. Laryngeal amyloidosis appears as an intermediate signal on T1 weighted MRI scan and low signal intensity on T2 weighted MRI scans similar to that of skeletal muscle. This is because protein fibrils of amyloid deposits lie in the form of parallel sheets similar to the organization of skeletal muscle fibres, therefore, can only be used in assisting a clinical and histopathological diagnosis of amyloidosis [4,7].
The management of localized laryngeal amyloidosis deposits centres on excision. There is a variation in the type of and the extent of surgery performed from the radical external approach to limited endoscopic laser excision of lesions. In our case, an endoscopic CO2 laser was used to excise the lesion surgically to good effect. However, as localized amyloidosis has been seen to recur, either locally or in a multi-focal manner, and rarely as a systemic disease, a surveillance programme must be recommended. In the case documented, amyloid deposits appear to have been causing functional impairment over a 7-year period until clinically apparent on direct laryngoscopy. The case, therefore, suggests that yearly follow-up should be undertaken for at least 10 years due to the nature of the disease [1,3,4,7–9].
Accurate diagnosis of localized amyloidosis and exclusion of systemic disease are crucial for correct management and prognosis. The treatment of localized amyloidosis is surgical and the use of endoscopic CO2 laser excision has been shown to be effective. Moreover, the slow progression of signs and symptoms of the disease in this case has demonstrated the need for follow-up over a number of years to ensure that recurrence is not missed.
Conflict of interest
None declared.
ACKNOWLEDGEMENTS
We are grateful to Dr. Z Ali for the provision of the histopathology images and the radiology department at Eastbourne District General Hospital for their contributions.



