-
PDF
- Split View
-
Views
-
Cite
Cite
D Papadopoulou, IP Chatziralli, V Papadopoulos, C Filitantzi, C Demertzidis, Synchronous gastric inflammatory myofibroblastic tumour with gastrointestinal stromal tumour of the stomach and hepatic syringious haemangioma, Journal of Surgical Case Reports, Volume 2012, Issue 1, January 2012, Page 7, https://doi.org/10.1093/jscr/2012.1.7
Close - Share Icon Share
Abstract
Inflammatory myofibroblastic tumour of the stomach is a very rare lesion. A case of a gastric inflammatory myofibroblastic tumour associated with gastrointestinal stromal tumour of the stomach and hepatic syringious haemangioma is described. We report an 80-year-old male who had an exophytic mass in the area of the pylorus and the duodenum, where hepatic cysts were found in the magnetic resonance (MRI) scan on examination of hypochromic microcytic anaemia, and prolapsus and torsion of the bulb of the stomach found during gastroscopy. During surgical excision of the exophytic mass, a gastrointestinal stromal tumour from the gastric fundus and a syringious haemangioma from the superior hepatic surface were resected. All tumours were treated successfully by surgical excision. The patient had an uneventful recovery. Neither recurrence nor metastasis was found after a 12-month follow-up. To our knowledge, this is the first time that such an association is reported in the literature.
INTRODUCTION
Inflammatory myofibroblastic tumour (IMT) is a very rare lesion. Inflammatory myofibroblastic tumours usually affect children and young adults, but can emerge at any age (1). IMT develops more often in the lungs (2). Extrapulmonary locations, such as mesentery, gastrointestinal tract, liver, gallbladder, spleen, genitourinary tract, upper respiratory tract, heart and breast, have been also described (3,4). The stomach presents a very rare localization of this tumour in adults, with few cases being reported in the international literature. Here, we present a case of gastric IMT in an adult concomitant with gastrointestinal stromal tumour of the stomach and hepatic syringious haemangioma. To our knowledge it is the first time that such a case is reported in the literature.
CASE REPORT
An 80-year-old man presented to our hospital with a 2-year history of cachexia, anorexia and weight loss. Physical examination was normal without presence of any pathological sign. Laboratory investigations were significant for hypochromic microcytic anemia (Ht 29.3; normal range 42-54). All tumour markers were negative.
The patient underwent investigation with gastroscopy, where torsion of the bulb of the stomach was found. The insertion of the instrument via the pylorus was not impossible (Figure 1). The upper gastrointestinal series disclosed polypous masses in the bulb and the prepyloric part of the stomach (Figure 2). The computerized tomography (CT) scan was unremarkable. The magnetic resonance (MRI) scan showed an exophytic mass with disturbance of the plication of the intestinal lumen in the area of the pylorus and the duodenum. Also, few small hepatic cysts were found.
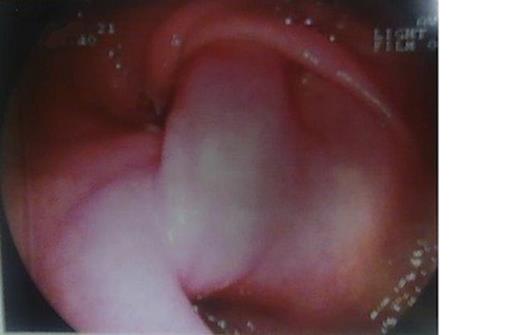
Esophagogastroduodenoscopic examination. Prolapsus and torsion of the bulb of the stomach.
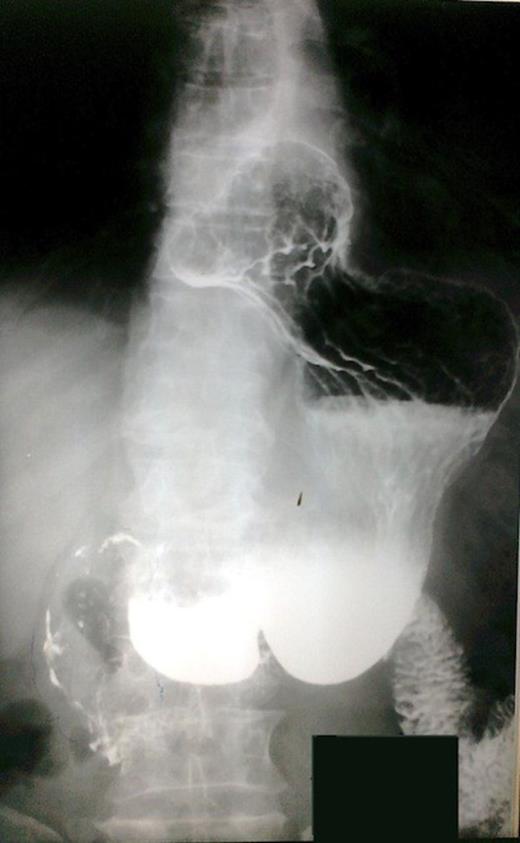
Upper gastrointestinal series. Polypous masses in the bulb and the prepyloric part of the stomach
During the laparotomy, a prepyloric intraluminal tumour was found, and a Billroth II resection was performed. In addition, a nodule from the anterior wall of the gastric fundus and a node from the superior hepatic surface were resected. Gross examination of the resected portion of the stomach revealed, in the mean part of the antero-superior surface of the stomach and 4cm above the inferior surgical edge, a projection of the mucosa with central crater, and underneath a tumour with submucosal localization was recognized. The tumour was well demarcated from the muscular coat and badly from the mucosa. Cut surfaces of the primary tumour showed a whitish gray mass with fibroelastic texture that was localized in the submucous coat. The tumour measured 3.5x3x2.5cm.
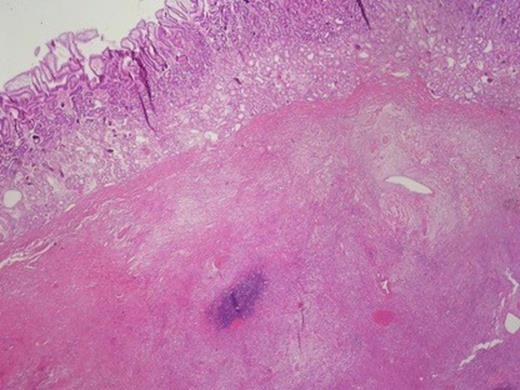
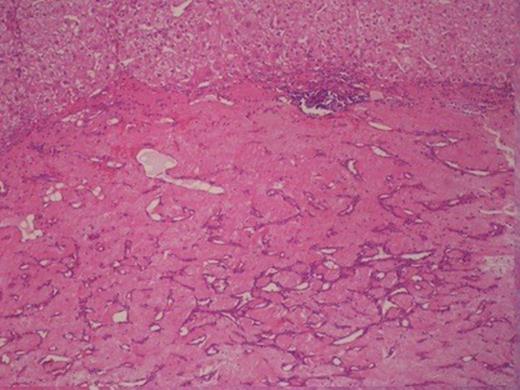
Histologically, the tumour was composed of fibroblasts that were arranged vertically. Smooth muscle cells were also recognized that apparently were remnants of the mucosal muscle layer. Also, redundant confluent lymphocytes, plasmocytes, mast cells with rare giant cell forms, as well as redundant eosinophilic granulocytes were recognized. The supernatant mucous membrane showed signs of active inflammation, and in the area of the crater, a healed ulcer was recognized. The final pathologic diagnosis was consistent with IMT that originated from the gastric wall (Figure 3).Gross examination of the nodule from the anterior wall of the fundus of the stomach showed a whitish gray elastic mass measuring 1.5x1x1cm. The tumour was identified as an incipient gastrointestinal stromal tumour that consisted of uniform neoplastic cells without nuclei atypia ((Figure 4).The node from the superior hepatic surface was identified as a syringious haemangioma. The dimensions of the haemangioma were 2x1.5x1cm and the tumour had chestnut complexion and friable texture ((Figure 5).
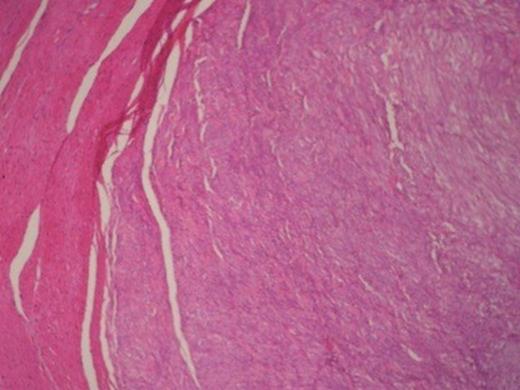
Gastrointestinal stromal tumour in the right and smooth muscle cells in the left.
The patient had an uneventful postoperative course and has been followed up for one year without any recurrence.
DISCUSSION
IMT is a very rare entity, whose aetiology still remains unknown. It is not completely declared if it is an actual tumour or severe inflammation and if it is malignant or benign (5). This condition is indicated by the fact that IMT is known with several terms, such as inflammatory pseudotumour, plasma cell granuloma, inflammatory myofibroblastoma and inflammatory myofibroblastic proliferation (3). According to the current classification of the World Health Organization, IMT is a neoplasm with a tendency for local recurrence and a very low rate of metastasis, and is histopathologically composed of myofibroblastic spindle cells, with inflammatory cell infiltrate of plasma cells, lymphocytes and eosinophils (6).
Even though IMT can be found in any site of the body and at any age, gastric IMT presents a very uncommon disease (5). Park et al have reported an exophytic gastric IMT as a cause of hemoperitoneum (1). Kim et al have also reported a gastric IMT with peritoneal dissemination in a young adult (7), while Al-Taie et al have described a rapidly growing gastric IMT following benign gastric ulcer (8). Moreover, Leon et al have found an IMT of the gastric remnant in a patient with a prior partial gastrectomy (9). Kojimahara et al have described a gastric IMT to the lesser curvature of the cardiac region in a young woman (10).
The variability of the clinical presentation and the radiological findings of this clinical entity make a preoperative diagnosis hard. A definitive diagnosis can be set only after the histological examination. The postoperative course is also unpredictable.
In conclusion, we have reported a rare case of a gastric IMT combined with a gastric gastrointestinal stromal tumour and hepatic syringious haemangioma. To our knowledge, this is the first case report in the international literature that combines the rare gastric IMT with the common gastrointestinal stromal tumour of the stomach and hepatic syringious haemangioma.



