-
PDF
- Split View
-
Views
-
Cite
Cite
A Kapoor, RK Singh, Periampullary carcinoma in a patient with agenesis of dorsal pancreas, Journal of Surgical Case Reports, Volume 2011, Issue 9, September 2011, Page 4, https://doi.org/10.1093/jscr/2011.9.4
Close - Share Icon Share
Abstract
Agenesis of dorsal pancreas is a rare developmental anomaly. We here report a case of agenesis of dorsal pancreas in a patient of periampullary carcinoma and highlight its implications on the management.
INTRODUCTION
Although rare, there is a wide spectrum of pancreatic malformations reflecting the complexity of pancreatic embryogenesis. Agenesis of the dorsal pancreas is a very rare pancreatic malformation. The first case was published as an autopsy finding in 1911 and till date, there are 54 descriptions of this entity in the English literature (1). We here report a case of agenesis of dorsal pancreas in a patient of periampullary carcinoma and highlight the implications of this embryological anomaly on the management.
CASE REPORT
A 55 year non-diabetic male with painless progressive jaundice, pruritus and weight loss of 3 month duration was admitted in emergency with cholangitis. Investigations revealed a total leukocyte count of 17 000/cu.mm, total bilirubin: 222.3 μmol/L, direct: 136.8 μmol/L and alkaline phosphatase level: 1153 U/L. Side viewing endoscopy showed an ulceronodular growth at papilla, a papillotomy and endoscopic biliary plastic stenting was done. Neither cholangiogram nor pancreaticogram was obtained in view of cholangitis. Once his cholangitis resolved, he was referred for surgery. Pre-operative triple phase CT (computed tomography) scan of the abdomen showed a resectable ill-defined hypodense mass in the region of ampulla, the pancreatic head and uncinate were normal however the neck, body and tail of pancreas were absent (figure 1,2).
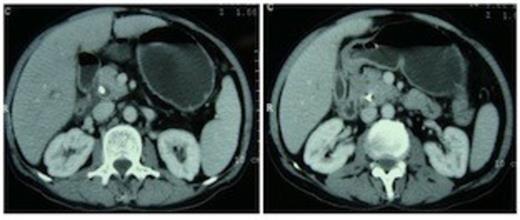
Computed tomography scan showing pancreatic head and uncinate process with absence of neck anterior to the portal vein. An ill-defined hypodense mass is seen in the ampullary region. Biliary stent is in situ.
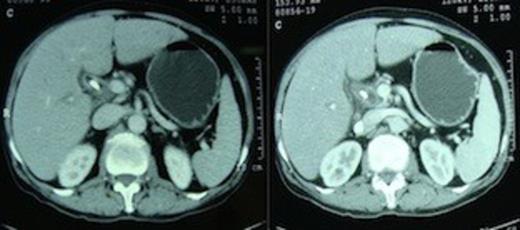
Computed tomography scan demonstrating absence of the body and tail of pancreas anterior to the splenic artery and vein.
Patient was counseled about the development of diabetes after pancreaticoduodenectomy, which for him would entail removal of the entire pancreas. At surgery, there was no dissemination, a 2×1 cm mass was felt in the region of ampulla. On opening the lesser sac the portal vein was lying bare and the pancreatic neck, body and tail were absent (figure 3).
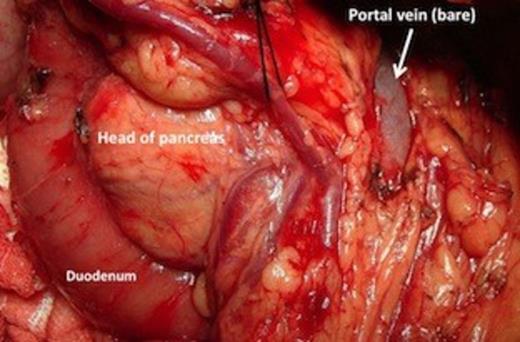
Operative picture showing the bare portal vein with absent neck, body and tail of pancreas.
The transverse mesocolon was intact with its anterior layer reflecting onto the lesser sac in the form of a peritoneal reflection covering the anterior aspects of splenic artery and vein. Only the head and uncinate process comprised the entire pancreas, which were normal in size and consistency with no evidence of any compensatory hypertrophy, enlargement or findings suggestive of acute or chronic pancreatitis. Resection was easy, with no need to create a tunnel as there was no pancreas overlying the portal vein and a pylorus preserving pancreaticoduodenectomy was done. Reconstruction comprised only of hepaticojejunostomy and duodenojejunostomy. The cut section of the resected specimen showed only the major papilla with a friable ampullary growth, there was no minor papilla (figure 4).
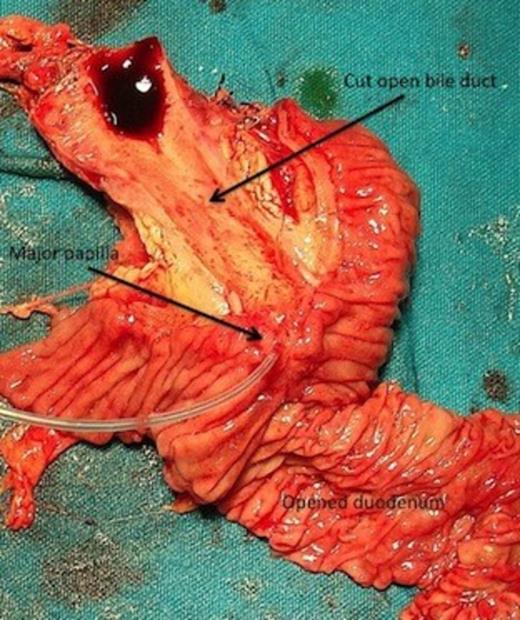
Pancreaticoduodenectomy specimen with longitudinally cut open medial wall of duodenum and bile duct showing only the major papilla (cannulated with a tube) with ampullary growth, minor papilla is absent.
A pancreatogram was done after cannulating the pancreatic duct through the major papilla. It demonstrated a single main ventral pancreatic duct with prominent side branches; no dorsal duct could be identified. Histopathology revealed an ampullary carcinoma infiltrating the superficial part of pancreas. The rest of the pancreatic tissue was normal with no features suggestive of acute or chronic pancreatitis. All the resection margins were free and none of the 14 lymph nodes had any metastasis. Postoperatively the patient was discharged on a fixed dose insulin regime and pancreatic enzyme replacement therapy.
DISCUSSION
Complete agenesis of the pancreas is incompatible with life (2). Agenesis of dorsal pancreas is the rarest congenital anomaly compatible with life (2). Pancreas develops from dorsal and ventral endodermal buds. The ventral bud rotates posteriorly and to the left, fusing with the dorsal bud during the seventh gestational week. The ventral bud differentiates into part of the head and uncinate process of pancreas. The neck, body, tail and superior aspect of head originate from the dorsal bud. The duct of Santorini represents the proximal part of the duct of the dorsal pancreas whereas the distal portion of the dorsal duct is retained as most of the main duct in body and tail regions (3). Agenesis of dorsal pancreas originates from a defect in embryogenesis and has been reported to be associated with other congenital anomalies (4,5).
W.J Schnedl et al systematically summarized all the 53 patients with agenesis of the dorsal pancreas reported in medical literature over the last 100 years and found diabetes mellitus to be most commonly associated disease with this malformation (6). Our patient did not have any history of preoperative hyperglycemia. Perhaps the normal pancreatic head and uncinate alone were sufficient in preventing development of any diabetes in this patient.
The diagnosis of agenesis of dorsal pancreas rests on the demonstration of the absence of the dorsal pancreatic duct. Agenesis of the dorsal pancreas may be partial (more frequent) or complete. In a complete agenesis, the minor papilla, accessory pancreatic duct, the body and tail of pancreas are absent. However in partial agenesis, the minor papilla with a remnant of the accessory pancreatic duct and a variable amount of pancreatic body are present (7). The diagnosis can be made by ultrasound, CT scan, magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP). Ultrasonography may fail to identify the abnormality because the bowel gas can obscure the details (8). CT scan can detect this entity, as only the pancreatic head is seen whereas the dorsal pancreas ventral to the splenic vessels is not seen (8). A pancreatogram (MRCP/ERCP) is essential to unequivocally diagnose partial or complete agenesis (9,10). In our case also the CT scan showed absence of dorsal pancreas. Although an MRCP or repeat ERCP would have conclusively diagnosed agenesis of dorsal pancreas preoperatively, these would not have changed the management. Therefore, instead of getting an MRCP or ERCP we obtained a pancreatogram by cannulating the pancreatic duct through the major papilla in the resected specimen. This pancreatogram served the purpose by clearly outlining the pancreatic ductal anatomy and established the diagnosis of complete agenesis of dorsal pancreas.
Pancreaticoduodenectomy in a patient with agenesis of dorsal pancreas would entail removal of all pancreatic tissue and hence result in postoperative endocrine and exocrine insufficiency requiring lifelong insulin and enzyme replacement therapy. The silver lining is the omission of a pancreatojejunostomy, which is considered to be the “Achilles heel” of this surgery.
CONCLUSION
Pancreaticoduodenectomy in patients with agenesis of dorsal pancreas entails removal of all the pancreatic tissue. Preoperative counseling is necessary because patients develop insulin dependent diabetes with exocrine insufficiency and require life-long replacement.



