-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca Zener, Yves Jacquet, John W. Wong, Danny Enepekides, Kevin M. Higgins, A rare case of surgical pathway implantation of clival chordoma presenting as a neck mass, Journal of Surgical Case Reports, Volume 2011, Issue 1, January 2011, Page 3, https://doi.org/10.1093/jscr/2011.1.3
Close - Share Icon Share
Abstract
Chordomas are rare, locally-aggressive tumours with a high rate of local recurrence. Recurrence along the route of surgical entry is an uncommon form of treatment failure. We report a case of a 59-year-old female who presented with a 3 cm neck mass in the left mid-sternocleidomastoid region. She had a history of a large clival chordoma resected via a transcervical, transparotid and transoral approach along with endoscopic intranasal exposure and a palatal split 4.5 years previously, followed by radiation to the primary site. Biopsy of the neck mass confirmed the diagnosis of chordoma recurrence following implantation in the surgical pathway. This case illustrates that while surgical pathway recurrence is a rare entity, it requires a high index of suspicion and should be considered in the differential diagnosis of a patient with a history of chordoma resection presenting with a mass more than two years after undergoing initial treatment.
INTRODUCTION
Chordomas are rare, slow-growing tumours that originate from ectopic notochordal remnants, found along the axial skeleton. (1) Chordomas originate in the sacral (29.2%), cranial (32%) and spinal (32.8%) regions. (2) Clival chordomas represent approximately half of cranial chordomas and they are more likely to occur in women and younger patients. (3) Even though they are histologically benign, chordomas are highly invasive and locally-destructive. Optimal surgical access and achieving complete resection often is constrained by the tumour’s close proximity to vital structures. (4,5) Even with adjuvant proton-beam radiotherapy, local recurrence rate is approximately 29% of skull base chordoma and is present in 95% of all treatment failure (6). An uncommon form of treatment failure is recurrence along the surgical entry route. Only three cases of a neck mass presenting as recurrence of the clival chordoma following implantation in the surgical pathway have been reported in the literature. Here, the fourth case of such an atypical occurrence is reported.
CASE REPORT
A 59-year-old woman presented with a gradually enlarging, asymptomatic neck mass. Past medical history was significant for clival chordoma resection and removal of a thyroglossal duct cyst. She had undergone resection 4.5 years prior via a transcervical, transparotid and transoral approach along with endoscopic intranasal exposure and a palatal split, followed by adjuvant radiotherapy. At the time of surgery, the incidental thyroglossal duct cyst was removed.
On examination, she had a 3 cm, firm and horizontally mobile neck mass intimate to her cervical incision line in the left mid-sternocleidomastoid region. FNA biopsy was performed and was consistent with chordoma. The patient underwent repeat staging with magnetic resonance imaging (MRI) of the head and neck (Figure 1), as well as computed tomography (CT) of the chest, abdomen and pelvis.
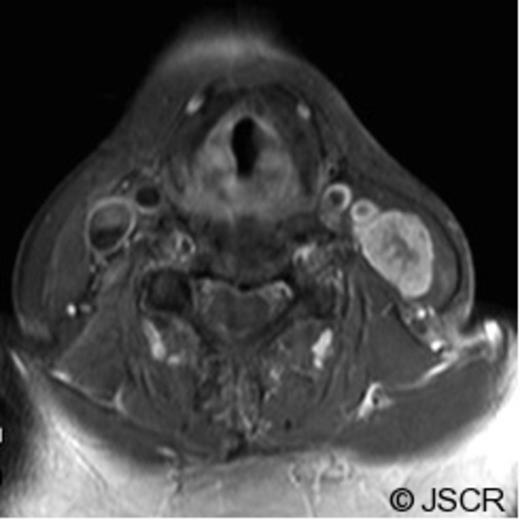
MR and CT Neck findings of the surgical pathway recurrence of a clival chordoma. (Axial, T1-weighted with Gadolinium MR image). Image obtained at the time of presentation with the neck mass, show a large, heterogeneous level III neck mass (23 mm x 17mm) with infiltration throughout the sternocleidomastoid muscle without obvious invasion of the great vessels. A few surrounding rounded lymph nodes within normal size limits are seen.
Contrast enhanced CT revealed some residual enhancement along the clival bone and left retropharyngeal area. The patient was reviewed at the multidisciplinary tumour board and with neurosurgery. She was diagnosed as having a recurrence of the chordoma following surgical pathway implantation. She underwent left salvage modified radical neck dissection (levels II-V) with sacrifice of the internal jugular vein and sternocleidomastoid, with preservation of the accessory nerve. Histopathology confirmed the presence of dermal fibrosis within the original neck scar. The left neck mass was composed of nests of large physaliferous cells with vacuolated cytoplasm surrounded by abundant myxoid material, consistent with chordoma (Figure 2). Surgical margins were clear. Lymph nodes were negative for malignancy. On immunohistochemistry, the tumour stained for low molecular weight keratin, high molecular weight keratin and epithelial membrane antigen. Stain for S100 protein was negative.
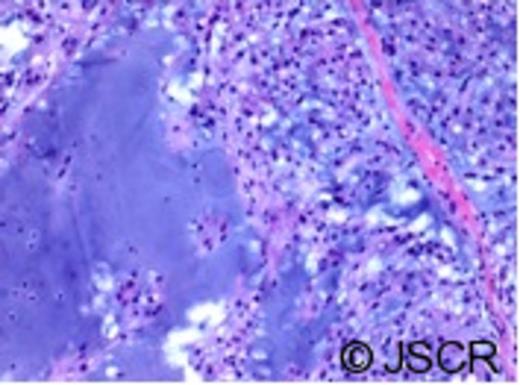
Histological appearance of chordoma. The tumour is composed of lobules of large cells with vacuolated cytoplasm admixed with a myxoid stroma.
DISCUSSION
The main differential diagnosis at the time of our patient’s presentation included distant metastasis, regional lymph node metastasis and surgical pathway recurrence. Recurrence along the surgical pathway is a distinct entity defined as a relapse situated along the surgical access route that is separated from the primary site of disease and outside of the marginal zone. It must be differentiated from a marginal recurrence, which arises in the area bordering the initial tumour volume, but yet immediately external to the prescribed radiation dose region. (5)
The rate of surgical pathway recurrence from a clival chordoma primary has been reported as 1.5%, and accounts for approximately 5% of all patients with chordoma recurrence after having undergone combined surgical and radiation therapy. (4) Fischbein et al. (2000) suggested that four criteria should be met in order to apply the term surgical pathway recurrence: i) disease found along the route of surgical entry to the original tumour resection; ii) presence of a minimum of 2.5 cm of normal intervening tissue between the disease and the site of the original tumour; iii) a minimum of 24 month interval between the time of surgery and recurrence; and iv) no evidence of perineural, lymphatic or distant metastases at the time of diagnosis. (4) In our patient’s case, all of these conditions were fulfilled. Using the aforementioned criteria for surgical pathway recurrence, only two other cases of clival chordomas with surgical implantation to the neck fulfilling these criteria have been reported. (6) Boyette et al. (2008) reported a case of recurrent clival chordoma presenting as a neck mass as a result of surgical pathway seeding after having undergone multiple resections and proton-beam irradiation for two local recurrences, where the primary resection involved insertion of harvested abdominal fat into the surgical bed with fibrin glue. (6)
It has been suggested that the infrequency of surgical pathway recurrence may be attributed to tumour cell non-viability secondary to physical damage and absent blood flow once separated from the main tumour mass. (7) Additionally, it has been suggested that with increased precision of radiation delivery to the tumour using more conformal proton-beams, the surgical pathway may not be covered by the radiotherapy delivered to the primary site. Furthermore, the neck is excluded altogether from the radiation field. Measures to prevent surgical implantation have been recommended including filling the operative tunnel with fibrin glue and cotton patties after completing the surgical approach, as well as changing instruments, gloves and contaminated towels and drapes prior to closure. (8) However, further investigation is required to determine the efficacy of filling the operative tunnel as a means of prevention.
Thought should be given to include the surgical pathway in the radiation field. Radiological follow-up assessment should include the surgical pathway relevant to the surgical approach in order to facilitate early detection of surgical pathway recurrence. Should a soft-tissue lesion be identified along the surgical pathway, the differential diagnosis would include a foreign body reaction to operative materials, postoperative granulation tissue formation and surgical pathway recurrence. Chordomas appear isointense or hypointense to brain on T1-weighted MRI. (9) Definitive diagnosis would require tissue biopsy; however, remarkable T2 hyperintensity on MRI would be redolent of recurrence. Surgical pathway recurrence is a rare entity requiring a high index of suspicion. Controlling recurrent disease within the surgical route is best accomplished through fastidious surgical resection and postoperative radiation. Even though surgical pathway recurrence may be controlled locally, since the majority of these cases experience primary or distant failure, they often have a poor prognosis.



