-
PDF
- Split View
-
Views
-
Cite
Cite
Anaan Fareed, Jordan Barrett, Ali Al-Mashat, Sanjaya Karunaratne, Small bowel obstruction from jejunal gastrointestinal stromal tumour in a young adult with neurofibromatosis type 1: a case report, Journal of Surgical Case Reports, Volume 2026, Issue 1, January 2026, rjag030, https://doi.org/10.1093/jscr/rjag030
Close - Share Icon Share
Abstract
Neurofibromatosis type 1 is an autosomal dominant disorder associated with an increased risk of tumours, including gastrointestinal stromal tumours. These are usually asymptomatic and discovered incidentally. Small bowel obstruction due to gastrointestinal stromal tumour in neurofibromatosis 1 is rare, especially in young adults. We report a 24-year-old man with neurofibromatosis type 1 who presented with right upper quadrant pain and biochemical features of acute cholecystitis. During laparoscopic cholecystectomy, unexpected small bowel dilatation prompted conversion to laparotomy, revealing obstruction from a large multifocal jejunal gastrointestinal stromal tumour. This case highlights the rarity of such a presentation and the importance of surgical adaptability intraoperatively.
Introduction
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder with a prevalence of ~1 in 3000 individuals [1]. It is characterized by café au lait macules, cutaneous neurofibromas and axillary freckling, and has a markedly increased lifetime risk of both benign and malignant neoplasms, with reported incidences ranging from 4% and 52% [2, 3]. Gastrointestinal manifestations occur in 10%–25% of NF1 cases, with gastrointestinal stromal tumours (GISTs) representing 7–25% of these lesions, most commonly arising in the small bowel, often the jejunum [3, 4]. Symptomatic small bowel obstruction (SBO) secondary to GIST in NF1 is rare. We present a case of an incidental jejunal GIST causing SBO identified intraoperatively during laparoscopic cholecystectomy for presumed acute cholecystitis, necessitating conversion to exploratory laparotomy. This case underscores the diagnostic challenges posed by NF1-associated intra-abdominal tumours and emphasizes the need for surgeons to maintain a broad intraoperative differential diagnosis and adapt operative strategies when unexpected findings arise.
Case presentation
A 24-year-old Caucasian man with known NF1 presented to a rural emergency department with a four-day history of right upper quadrant pain radiating to both shoulders, accompanied by nausea, vomiting, and subjective fevers. Symptoms commenced following ingestion of a high-fat meal. His medical history was notable only for NF1, with no prior abdominal operations. He was a lifelong non-smoker and reported minimal alcohol consumption.
On transfer to our facility, the patient was hemodynamically stable (blood pressure 130/70 mmHg, heartrate 76 bpm, afebrile). Abdominal examination demonstrated right upper quadrant tenderness without peritonism. Laboratory investigations revealed leucocytosis (12.2 × 109/L) with neutrophilia (9.3 × 109/L), a mildly elevated C-reactive protein (6 mg/L), and deranged liver function tests (GGT 144 U/L, ALP 126 U/L, ALT 157 U/L, and AST 56 U/L) with a normal bilirubin. Renal function and electrolytes were within normal limits.
Abdominal ultrasound confirmed acute cholecystitis with cholelithiasis, gallbladder wall thickening (5.7 mm), and a positive sonographic Murphy’s sign. He was commenced on intravenous ceftriaxone 2 g daily and metronidazole 500 mg BD. On Day 2 of admission, due to escalating pain, he was taken for urgent laparoscopic cholecystectomy.
Intraoperatively, the small bowel was found to be markedly dilated and tense, with reduced peristalsis (Fig. 1), findings consistent with small bowel obstruction. Visualization was aided by the laparoscope light. Given the degree of distension and concern regarding the underlying aetiology, a second opinion was obtained from another consultant general surgeon. Decision was made to convert to an exploratory laparotomy. Informed consent was obtained from the patient’s next of kin.
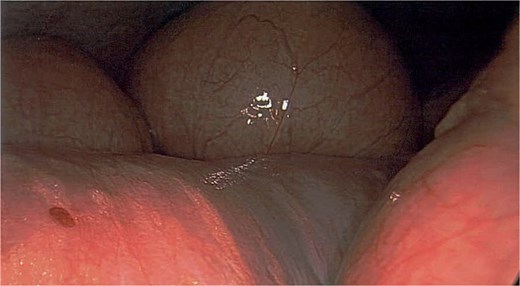
Dilated small bowel loops during laparoscopy. Visualization aided by standard laparoscope illumination.
During laparotomy, a pedunculated, cream-coloured mass measuring 10 × 3 cm was identified on the antimesenteric border of the proximal jejunum, with multiple smaller nodules present on the adjacent serosal surface. The affected jejunal segment containing the mass and nodules was resected, and a retrograde cholecystectomy was subsequently performed.
Macroscopic examination revealed a 90-mm jejunal mass with cystic, necrotic, and haemorrhagic components, accompanied by 13 smaller nodules (1–7 mm) (Figs 2 and 3) The gallbladder demonstrated cholecystitis and contained ~20 gallstones (5–15 mm). Microscopically, the jejunal lesion represented a multifocal spindle-cell GIST involving up to 14 foci. The largest focus measured 90 mm, with a mitotic index of 4 per 5 mm2 and ~50% necrosis. Resection margins were clear, and no lymphovascular invasion or tumour rupture was identified. Ten reactive lymph nodes were negative for metastatic disease. Immunohistochemistry demonstrated strong positivity for CD117, DOG1, CD34, and SDHA/SDHB, consistent with an NF1-associated GIST with wild-type KIT/PDGFRA profile.
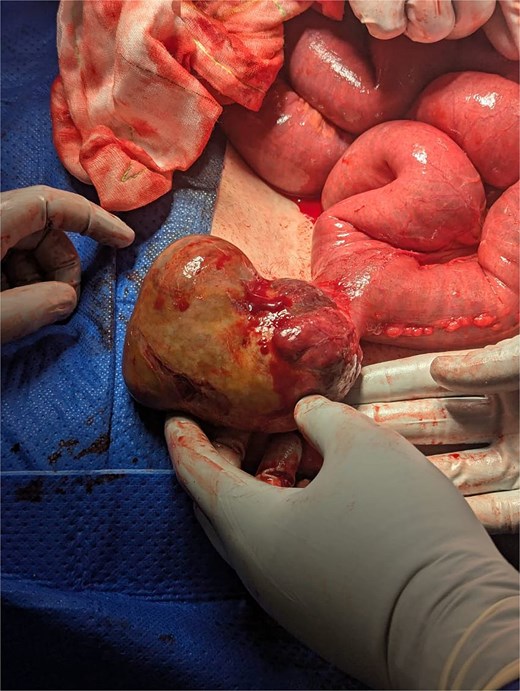
Intraoperative image of GIST arising from the antimesenteric border of jejunum, along with dilated small bowel loops.
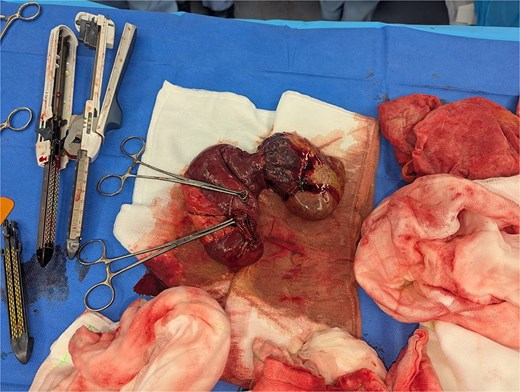
Postoperatively, the patient developed a prolonged ileus requiring total parenteral nutrition but subsequently achieved full recovery. He was referred to medical oncology and commenced on adjuvant imatinib 400 mg daily with a planned duration of 3 years.
Discussion
This case highlights an uncommon and diagnostically challenging scenario where a jejunal GIST causing SBO was discovered incidentally during laparoscopic cholecystectomy for presumed acute cholecystitis. The patient’s clinical presentation, including right upper quadrant pain, postprandial symptoms, deranged liver function tests and ultrasound findings, aligned with typical features of acute cholecystitis. However, intraoperative recognition of dilated small bowel loops prompted conversion to exploratory laparotomy, ultimately revealing and permitting resection of the obstructing jejunal tumour.
Although gastrointestinal manifestations are observed in 10%–25% of patients with NF1, the majority remain clinically silent, and only a minority present with complications such as obstruction [3, 5]. When obstruction occurs, GISTs are recognized as the second most common cause after neurofibromas [3]. SBO due to NF1-associated GIST is rare, particularly in young patients without prior abdominal surgery. Schaeffer et al. and Saha et al. described similar cases in older individuals (38 and 65 years, respectively), while Miettinen et al. reported a median age of 49 years for NF1-associated GISTs, most frequently presenting with gastrointestinal bleeding or anaemia [6–9].
NF1-associated GISTs differ from sporadic counterparts in several respects: they typically arise in the small intestine, are often multifocal, and occur at a younger age [3, 5]. They are generally wild-type for KIT and PDGFRA mutations, although they usually demonstrate immunopositivity for CD117 and DOG1. While these tumours are often regarded as indolent, risk stratification remains challenging, particularly in multifocal disease. While NF1-associated GISTs are often regarded as relatively indolent, risk stratification remains challenging, particularly in the setting of multifocal disease. Prognostic assessment is typically guided by modified NIH/AFIP criteria, which incorporate tumour size, mitotic index, and anatomical site [10]. In this case, the tumour demonstrated several adverse features including large size (90 mm), elevated mitotic activity (4 per 5 mm2), and extensive tumour necrosis (~50%), thus supporting a high-risk classification and justifying the decision to initiate adjuvant imatinib despite the wild-type KIT/PDGFRA genotype.
Although preoperative cross-sectional imaging was not obtained in this case given the convincing ultrasonographic findings of acute cholecystitis, contrast-enhanced can be valuable in NF1 patients with abdominal pain to evaluate the wider spectrum of intra-abdominal manifestations and may identify incidental small-bowel GISTs that are otherwise clinically occult.
This case also underscores the potential for overlapping or misleading clinical presentations in NF1. Symptoms attributable to common conditions, such as cholecystitis or biliary colic, may obscure underlying syndromic pathology. Consequently, surgeons should maintain a broad differential diagnosis when evaluating NF1 patients with abdominal complaints.
Conflicts of interest
The authors declare no conflicting interests.
Funding
No external funding was obtained for this case report.
Consent to participate and consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.



