-
PDF
- Split View
-
Views
-
Cite
Cite
Badria Eid Aljohani, Duaa Salem Alkhayat, Rouz Faisal Abu Sulami, Abdullah Khalid Aljohani, Mammary malignant peripheral nerve sheath tumor in a 93-year-old male: case report, Journal of Surgical Case Reports, Volume 2026, Issue 1, January 2026, rjag029, https://doi.org/10.1093/jscr/rjag029
Close - Share Icon Share
Abstract
Breast sarcomas are rare malignancies, accounting for <1% of all breast malignancies and <5% of all soft tissue sarcomas. Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive soft tissue sarcomas that arise from peripheral nerves. Their occurrence in the breast is exceedingly rare. This is the fourth published case of mammary MPNST in a male patient. We present a case of a 93-year-old male with a three-year history of a progressively enlarging and painful right breast mass. Examination revealed a 20 × 30 cm immobile, fungating, and ulcerating mass with purulent discharge. Histopathological assessment confirmed a high-grade spindle cell sarcoma, and immunohistochemistry revealed positivity for vimentin, S100 protein, and p63 in a focal pattern, confirming the diagnosis of MPNST. The patient underwent a right mastectomy, achieving a surgical clearance. After 3 months, he passed away with malignant pleural effusion due to lung metastasis.
Introduction
Breast sarcomas are extremely rare malignant tumors originating from mesenchymal tissue, accounting for <1% of all breast malignancies and <5% of all soft tissue sarcomas [1]. Malignant peripheral nerve sheath tumors (MPNSTs) are rare aggressive soft tissue sarcomas arising from peripheral nerves or demonstrating nerve sheath differentiation. Accounting for 5%–10% of all soft tissue sarcomas, with an incidence of 1:100 000. They are commonly associated with neurofibromatosis type 1 (NF1), but they can appear de novo. MPNSTs commonly involve the trunk (51%) and extremities (45%); their occurrence in the breast is exceedingly rare [2]. Adding to the diagnostic challenge, they are particularly uncommon in males. To our knowledge, there are only three published case reports of mammary MPNSTs in male patients [3–5]. This case report discusses a 93-year-old male with high-grade MPNST, focusing on the clinical presentation, diagnostic approach, and surgical management.
Case report
A 93-year-old male with no significant medical or surgical history presented to the oncology clinic with a painful right breast mass that had been progressively enlarging over the past three years, accompanied by worsening pain over the last three months. He reported no history of radiation exposure or family history of malignancies. On local examination, there was a 20 × 30 cm fixed mass in the right breast, adherent to the chest wall, fungating, ulcerated, with purulent discharge, and no palpable axillary lymphadenopathy (Fig. 1). Additionally, a large, soft, and mobile mass was noted on the right upper back, present for over 15 years, with normal overlying skin, which was clinically and radiologically suggestive of a lipoma.
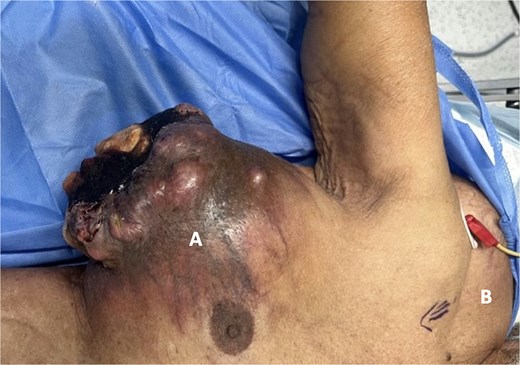
Preoperative picture. (A) Right breast mass, immobile, fungating, and ulcerating with purulent discharge. (B) Right upper back mass, soft, mobile, with normal overlying skin, suggestive of lipoma.
Laboratory investigations, including a complete blood count, liver function tests, kidney function tests, and electrolytes, were all within normal limits. Imaging studies provided more insight into the patient's condition. Breast ultrasound revealed a heterogeneous mass, inferior and lateral to the right breast, measuring 8.1 × 7.5 cm. The mass was semisolid and partially compressible, with internal cystic spaces and vascularity. Unenhanced computed tomography (CT) of the chest demonstrated a heterogeneous mass lesion in the right breast, measuring 8.0 × 8.7 cm, with hyperdensities and no calcifications, extending from the mid to anterior axillary line. Additionally, there was a large, fat-density lesion in the upper back, suggestive of a lipoma. There was no evidence of distant metastases (Fig. 2).
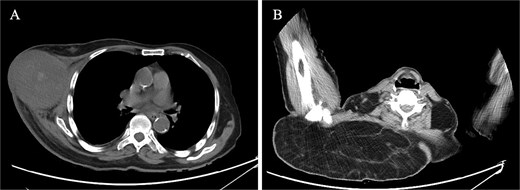
CT findings. (A) CT scan of right breast showing heterogeneous mass, measuring 8.0 × 8.7 cm, extending from the mid to the anterior axillary line. (B) CT scan of right upper back showing a large, fat-density mass, suggestive of lipoma.
Histopathological examination of a core needle biopsy revealed a malignant spindle cell neoplasm. The neoplasm was characterized by alternating hypercellular and hypocellular areas, with spindle cells arranged in short fascicles around central vascular channels. The overlying tissue showed ulcerated keratinizing squamous epithelium with marked acute inflammation. There was no breast tissue identified.
The immunohistochemistry results revealed several key markers in the tissue sample. Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, cytokeratin 7, CK 5/6, epithelial membrane antigen, smooth muscle actin, desmin, CD34, and beta-catenin all tested negative, suggesting that the tumor is not of epithelial or hormone receptor-positive origin, nor is it a smooth muscle or endothelial-related tumor. Conversely, the tumor showed positive staining for vimentin, a marker for mesenchymal cells, indicating a sarcomatous origin. S100, a marker for neural and melanocytic tumors, showed strong staining in a focal pattern, further supporting the neural differentiation of the tumor and the diagnosis of the MPNST. p63, a marker for squamous cell differentiation, showed focal positivity, indicating some squamous component in the tumor. Ki-67, a marker of cell proliferation, exhibited a high proliferation index, suggesting rapid tumor growth. Based on these findings, the tumor was diagnosed as a spindle cell sarcoma, with a strong favor for MPNST.
The patient underwent a right mastectomy, along with an excision of the posterior neck lipoma. During surgery, the tumor was found to involve the lateral border of the breast, attaching to the pectoralis muscle and latissimus dorsi laterally, and extending into the thoracodorsal bundle. There was no lymph node dissection. To close the surgical defect, a local rotational skin flap was utilized (Fig. 3). Postoperative histopathological examination revealed a high-grade sarcoma composed of pleomorphic spindle cells arranged in fascicles, with honeycomb-like infiltration into the subcutaneous fat and muscle. The overlying skin was ulcerated, showing marked atypia and frequent mitotic activity.
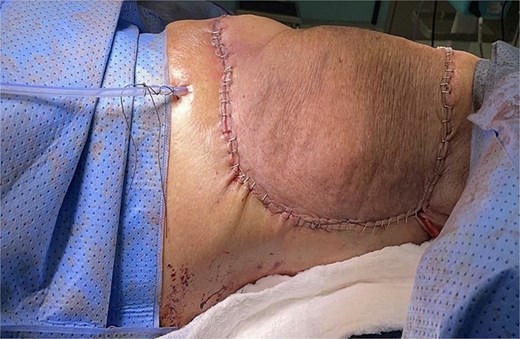
Postoperative picture. Following the right mastectomy and excision of the right upper back lipoma, a local rotational skin flap was utilized to close the surgical defect.
The tumor was classified according to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) as Grade 3 (Score 6), with a breakdown of 2 points for tumor differentiation, 1 point for necrosis, and 3 points for mitotic activity. The tumor has a pathological stage of T4, indicating direct extension of the tumor to the chest wall or skin. Immunohistochemistry results were consistent with the preoperative biopsy. The histopathological examination of the posterior neck mass revealed a spindle cell lipoma. Postoperatively, there were no immediate complications. After 3 months, he passed away with malignant pleural effusion due to lung metastasis.
Discussion
Imaging modalities lack specificity for MPNSTs. Most mammary sarcomas appear hypoechoic without posterior acoustic shadowing on ultrasound and as heterogeneous masses with irregular margins on magnetic resonance imaging. On mammography, it appears as a hyperdense mass with indistinguishable or circumscribed margins, with or without calcifications or spiculated lesions [6].
The diagnosis of MPNSTs relies mainly on histopathology and immunohistochemistry. MPNSTs have a morphological similarity to fibrosarcomas, as they are composed of spindle-shaped cells within a myxoid matrix [7]. Immunohistochemistry should show positivity for S100 protein in a focal pattern; otherwise, if it is diffuse, it suggests the diagnosis of cellular schwannoma or metastatic melanoma [8]. Differential diagnoses of the mammary MPNSTs include phyllodes tumors, metaplastic breast carcinomas, inflammatory carcinomas, and mammary lymphomas [7].
Total mastectomy is the gold standard for breast sarcomas. Axillary regional node dissection is not indicated, as the mode of dissemination of sarcomas is primarily hematogenous. The role of adjuvant chemotherapy and radiotherapy is not well-established, but may be considered in cases with metastatic disease and inadequate surgical margins [2].
Soft tissue sarcomas have a generally poor prognosis; 25% of patients develop distant metastasis even after curative surgical resection of the primary tumor. Metastases occur in the lung in 70% of cases [8].
Conflicts of interest
None of the authors has any conflicts of interest to disclose concerning this article.
Funding
None declared.



