-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah E Kim, Daniel Steeno, Hassan A Qureshi, Alexander P Lynch, Francis J Podbielski, Post-lobectomy lung abscess, Journal of Surgical Case Reports, Volume 2026, Issue 1, January 2026, rjaf1087, https://doi.org/10.1093/jscr/rjaf1087
Close - Share Icon Share
Abstract
A residual lung parenchyma abscess following pulmonary resection for lung cancer is a rare and potentially serious condition. We present a case of successful nonoperative management of a lung abscess that developed after a right upper lobectomy for adenocarcinoma in a 76-year-old man who experienced systemic symptoms 6 weeks after his initial surgery. Imaging revealed an abscess in the previous surgical cavity. We discuss management strategies and challenges associated with this uncommon condition.
Introduction
Lung abscess following lobectomy is a rare complication that can cause significant morbidity and mortality. A 76-year-old man underwent right upper lobectomy for stage IIIA pulmonary adenocarcinoma and presented 2 months post-surgery with systemic symptoms. Cross-sectional imaging was diagnostic for an abscess in the surgical bed. We review the treatment modalities and considerations when addressing this complication.
Case report
We present a 76-year-old male with squamous cell carcinoma of the scalp, paroxysmal atrial fibrillation, prior pulmonary embolism, and biopsy-proven stage IIIA pulmonary adenocarcinoma, who completed neoadjuvant chemotherapy and immunotherapy prior to surgical resection. He underwent a right thoracotomy and right upper lobectomy with extensive adhesiolysis to enable lymphadenectomy in the R2 and R4 stations, which were adherent to the superior vena cava, aorta, and right main pulmonary artery. The lobectomy was carried out in a standard manner and the bronchial stump was reinforced with an intercostal muscle flap. The patient recovered uneventfully and was discharged.
Six weeks postoperatively, he presented to the emergency room with several weeks of chest pain, dyspnea, and fatigue. A non-contrast computed tomography (CT) scan revealed a large cavitary mass of the posterior right upper lobe hilum, associated with fluid collections in the adjacent chest wall and loculated pleural effusion, raising concern for cavitary abscess (Fig. 1). He was admitted to the hospital floor and started on broad-spectrum intravenous antibiotics.
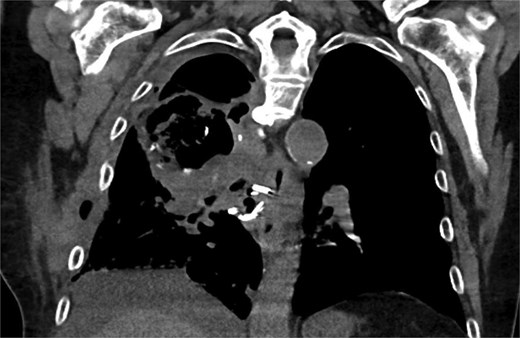
Coronal CT showing large cavitary lesion in the right upper lobe measuring 10.0 × 6.0 cm, loculated right pleural effusion, complex chest wall fluid collection.
In consultation with the interventional radiology team, the patient underwent image-guided pigtail catheter placement for the pleural effusion and a second drain for the anterior chest wall fluid collection. The patient’s pleural cultures grew Lancefieldella parvula, an oral flora, with negative CT imaging of the face. A bronchoscopy was performed showing copious secretions in the right lung, without evidence of bronchial stump leak.
Subsequently the patient showed clinical and radiographic improvement, allowing pleural catheter removal. Surveillance CT scans at 2 and 6 months after hospitalization showed a decrease in the size of the intrapleural abscess without malignant recurrence (Fig. 2).
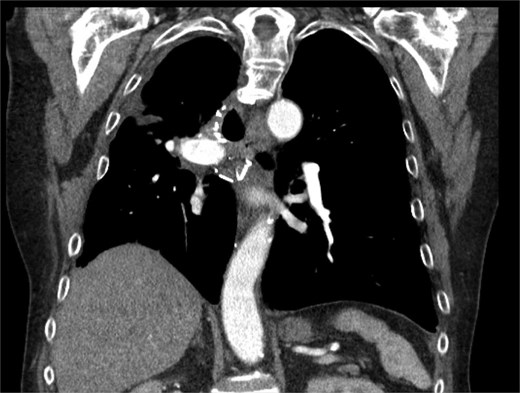
CT chest at 6 months with contrast with trace loculated pleural effusion and improved consolidation.
Discussion
Common pulmonary complications following lobectomy include pneumothorax, prolonged air leak, pneumonia, pleural effusion, atelectasis, hemothorax, and bronchopleural fistula. Abscess formation in the remaining lobe(s) after lobectomy is rare, with an incidence of <1%.
The risk factors for lung parenchyma include active smoking, chronic obstructive pulmonary disease, and the absence of regional anesthesia. Clinical markers may include fever, leukocytosis, and early opacification with progressive consolidation on plain chest X-ray. This can be confirmed on CT imaging and may be characterized with bronchoscopy, which allows for lavage to narrow antibiotic therapy. Typical bacterial pathogens are often found in the oropharynx; thus, oral hygiene and pulmonary toileting are critical for prevention of this complication [1].
Additional techniques for drainage of lung abscesses include endoscopic catheter drainage and percutaneous transthoracic tube drainage [2]. One study included 42 patients with lung abscess (secondary to pneumonia, tuberculosis, lung cancer, or aspergilloma) who had failed antibiotic therapy and subsequently underwent endoscopic catheter drainage of the abscess and were flushed daily with antibiotics [3]. Out of 42 patients who successfully underwent catheter placement and antibiotic lavage, 38 (91.9%) experienced significant clinical improvement, evidenced by a notable reduction in abscess size. A meta-analysis of 26 studies (194 patients) found 86.5% improvement after CT-guided percutaneous tube drainage, supporting minimally invasive drainage as an effective approach [4].
Surgical intervention for lung abscess has been documented to have a mortality as high as 11%, which may be influenced by the underlying circumstances, such as comorbid conditions that are often seen in patients who require surgical intervention [5]. Because of this, surgical intervention of lung abscess is typically reserved for patients with one of the following: massive hemoptysis, prolonged sepsis, bronchopleural fistula, or refractory response to antibiotics and nonoperative management such as percutaneous drainage. A prior study reviewed mortality rates in 91 patients who had undergone surgical intervention for infectious lung abscesses [6]. Thirteen out of 91 patients had mortality within 30 days, and significant predictors for fatal outcome included pulmonary etiology of sepsis, air leak, and organ failure. Patients had received either a segmentectomy, lobectomy, or pneumonectomy; of note, there was no difference in mortality between the three operations.
Similarly, lung abscesses in residual lung parenchyma after oncologic lobectomy have also been observed, often requiring operative management. In a large-scale, single-center study involving 1460 patients who underwent elective pulmonary lobectomy or bilobectomy for non-small cell lung cancer, only five patients were found to have complications related to abscesses in residual lung parenchyma [7]. Notably, none of the patients underwent neoadjuvant treatment, and all were reported to have otherwise uncomplicated intraoperative courses. They were extubated immediately after surgery, received appropriate surgical prophylactic antibiotics, and participated in physiotherapy. Interestingly, the abscesses developed relatively soon after the surgery, with diagnoses made around postoperative Day 8 after having signs and symptoms of obstructive pneumonia. Among those affected, 80% required repeat thoracotomy, while the remaining 20% could not undergo surgery due to being unable to tolerate the procedure. This study recommended surgical resection as the preferred treatment.
However, our patient did not need to undergo operative intervention as he had clinical improvement with systemic antibiotics and drainage. Bronchoscopies are used to perform both therapeutic and diagnostic interventions. Additionally, given remote presentation of the lung abscess as well as his nontoxic course with imaging and clinical response, our patient was able to avoid the risks of repeat thoracotomy.
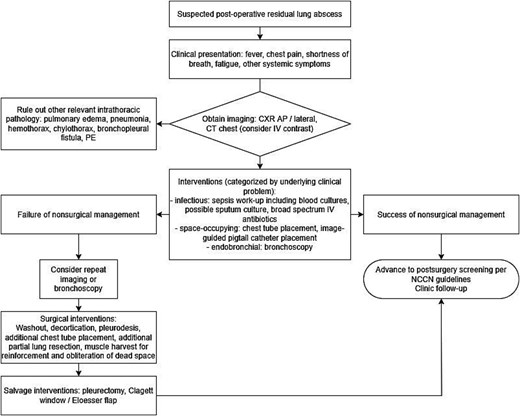
Lung abscess after oncologic resection is a rare complication with the potential for high morbidity and mortality. This case presentation of postoperative lung abscess, manifested many weeks after the index surgery, highlights the significance of early recognition through cross-sectional imaging. While surgical resection remains an option for patients unresponsive to conservative measures, our case demonstrates successful non-operative management with antibiotics and image-guided drainage. Our case also suggests the importance of a step-up, goal-directed approach to treatment: early diagnostic imaging, cross-sectional imaging-guided abscess drainage with cultures to drive antibiotics, and consideration of endobronchial visualization to minimize morbidity from surgerized wound beds such as bronchial stumps (Fig. 3).
Conflict of interest statement
None declared.
Funding
None declared.



