-
PDF
- Split View
-
Views
-
Cite
Cite
Aditya Shiva Rudraiah, Pratik Raichurkar, Anil Keshava, Metastatic prostate cancer mimicking colorectal cancer: a rare case of peritoneal and mesocolic lymph node involvement, Journal of Surgical Case Reports, Volume 2026, Issue 1, January 2026, rjaf1076, https://doi.org/10.1093/jscr/rjaf1076
Close - Share Icon Share
Abstract
Metastatic prostate cancer seldom involves the gastrointestinal tract, with simultaneous peritoneal and mesocolic nodal spread and is unreported in literature. We describe the case of a 93-year-old man with stable metastatic prostate cancer who presented with sigmoid obstruction secondary to sigmoid adenocarcinoma. Pathological assessment of the specimen confirmed sigmoid adenocarcinoma as well as prostate cancer deposits in colonic serosa, peritoneum, and mesocolic nodes that was not evident on preoperative imaging. This case highlights a previously undocumented metastatic pattern, the limitations of conventional imaging, and the importance of intra-operative vigilance in dual malignancies.
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed malignancies in men. Whilst it predominantly metastasises to the bone (84%), distant lymph nodes (10%), and liver (10%) [1], involvement of the gastrointestinal tract is exceedingly rare, accounting for ˂3% of cases [2]. Metastatic deposits in the colon, peritoneum, or colonic mesentery are especially uncommon, with only a handful of cases described in the literature [3–7]. We present a rare case of a 93-year-old male with a known history of metastatic prostate cancer who was found to have synchronous primary sigmoid adenocarcinoma and previously undiagnosed peritoneal and mesocolic lymph-node metastases from prostate cancer. To our knowledge, this is the first reported case demonstrating both peritoneal and mesocolic lymph-node involvement by prostate cancer, masquerading as colorectal cancer (CRC).
Case report
A 93-year-old male presented to the emergency department with a two-day history of worsening abdominal pain and constipation. His medical history was notable for metastatic prostate cancer with bony disease, managed with androgen-deprivation therapy alone. A recent prostate-specific-antigen (PSA) test suggested stable disease at 20 ng mL−1.
Computed tomography (CT) of the abdomen and pelvis revealed a partially obstructing apple-core lesion in the sigmoid colon suspicious for malignancy (Fig. 1). Multiple small hepatic hypodensities and a sclerotic focus in the L4 vertebra were also noted; staging CT of the chest showed no pulmonary metastases.
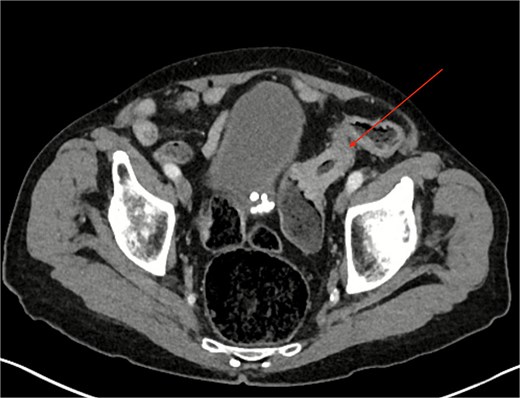
Colonoscopy after bowel preparation was attempted, but tight angulation and stricturing prevented stenting and a decision was made to perform an anterior resection for oncological control and to prevent obstruction.
Following multidisciplinary optimization, the patient underwent an uneventful laparoscopic high anterior resection by an experienced colorectal surgeon (A.K). Intra-operatively a large, bulky sigmoid tumour with serosal involvement was noted. Low-volume peritoneal disease, with multiple sub-5 mm nodules on the pelvic peritoneum and the bladder peritoneum, were also seen. This distribution was consistent with colorectal peritoneal metastases (CPM). A laparoscopic high anterior resection with high ligation of the inferior mesenteric artery, pelvic peritonectomy, and double stapled colorectal anastomosis was successfully performed.
The patient recovered well under a modified Enhanced Recovery After Surgery protocol and was discharged on the 6th postoperative day. Histopathology showed a primary sigmoid adenocarcinoma (pT3 N0). Metastatic prostate adenocarcinoma involved the colonic serosa, four of 17 mesocolic lymph nodes and the excised peritoneal lesions. No colorectal carcinoma was present in those lymph nodes or peritoneal specimens.
Postoperative multidisciplinary review concluded that, given adequate lymph node yield and complete resection achievement for CRC, adjuvant therapy was not required for this pathology. However, due to disseminated prostatic malignancy, the patient underwent reassessment, and consideration of systemic therapy for PCa.
Discussion
Metastatic prostate cancer to the gastrointestinal tract is rare. A large population-based analysis found that ˂3% of patients with advanced PCa develop metastases to this region [1]. Isolated case reports have described colonic involvement [2, 4], whereas peritoneal carcinomatosis from prostate cancer is exceedingly uncommon, with one such case identified on imaging alone via ^68Ga-PSMA Positive Emission Tomography (PET)/Computed Tomography (CT) imaging [5]. To our knowledge this is the first documented case demonstrating both peritoneal and mesocolic lymph-node involvement from PCa, which macroscopically mimicked CPM.
CRC typically spreads to the colonic serosa in advanced (T4) disease and to regional lymph nodes. Through tumour-cell shedding and peritoneal fluid dynamics, approximately 5% of T4 CRC cases present with synchronous peritoneal metastases [8, 9]. The intra-operative management of incidentally discovered synchronous CPM remains controversial; however, resection of limited synchronous CPM offers a survival benefit [10]. In the present case, presumed T4 CRC with pelvic peritoneal deposits was encountered. Given the patient’s age, frailty and ineligibility for intraperitoneal chemotherapy or a second-look procedure, the lesions were resected through a pelvic peritonectomy with the primary tumour. Pathology revealed only localized CRC and extensive metastatic PCa.
Current imaging techniques have limitations in detecting both peritoneal and pathological mesenteric lymph nodes. Initial staging CT did not detect extracolic disease, reflecting the modality’s modest sensitivity for peritoneal lesions below 1 cm (50%) and only 11% below 0.5 cm [11]. The sensitivity of CT imaging is further reduced when pathological nodes are not enlarged [12].
Whilst radioactive tracer enhanced bone scans and CT remain first-line imaging modalities for metastatic PCa, advanced imaging such as PSMA PET/CT has greater efficacy in detecting lymph nodes metastases [12]. In an Australian multicentre trial, PSMA PET/CT showed greater sensitivity (85% vs 38%) and specificity (98% vs 91%) than conventional imaging for staging high-risk PCa [13].
Prostate-specific antigen (PSA) remains the most widely used screening and monitoring tool for PCa. Though highly sensitive, it lacks specificity, and surveillance protocols vary based on risk stratification, with PSA commonly used to identify progression of metastatic disease [14]. This patient had stable PSA measurements and known high volume metastatic disease, managed with androgen deprivation therapy and surveillance. However, there is limited guidance in surveillance imaging for patients with metastatic hormone sensitive prostate cancer (mHSPC). The American Urological Association recommends periodic imaging for patients with mHSPC regardless of PSA trends, however there are no formally agreed upon surveillance intervals, with recommendations being for conventional imaging techniques only [15]. Newer imaging techniques including PMSA PET should be incorporated into future guidelines to help improve surveillance of mHSPC. In the current case, our patient had stable metastatic disease confined to the bone on imaging performed over 1 year prior to presentation, with the findings of peritoneal and lymphatic spread being discovered incidentally during surgery for his concurrent colonic malignancy.
Given the patient's advanced age and comorbidities, aggressive systemic therapy may not be feasible. Nonetheless, the incidental discovery of further metastatic disease warrants careful re-evaluation by specialist uro-oncologists.
Conflict of interest statement
None declared.
Funding
None declared.
References
Abulawi A, Azem A, Khatim I, et al. Prostate cancer with colonic metastasis: a rare case report. Am J Gastroenterol 2022;



