-
PDF
- Split View
-
Views
-
Cite
Cite
Lukas Sommer, Sidney Heersche, Sergio Gaspar-Figueiredo, Olivier Martinet, Acute abdomen in a young patient: an uncommon and difficult diagnosis of primary Group A streptococcal peritonitis with toxic shock syndrome, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf779, https://doi.org/10.1093/jscr/rjaf779
Close - Share Icon Share
Abstract
Primary peritonitis due to a Streptococcal Group A infection is a rare condition which is challenging to accurately diagnose due to a vast clinical presentation ranging from abdominal pain to septic shock, sometimes complicated by Toxic Shock Syndrome. Prompt blood cultures, supportive treatment, and antibiotic therapy are the mainstay of treatment. Surgical exploration can be necessary in case of clinical deterioration and suspicion of secondary peritonitis. The aim of this article is to address the clinical characteristics, challenges in diagnosis, and management options for this potentially fatal syndrome.
Introduction
Streptococcal of Lancefield Group A (GAS) β-hemolytic infections are common among young patients, typically causing skin, pulmonary, or oropharyngeal infections [1]. The most severe scenario is progression to Toxic Shock Syndrome, defined by hypotension associated with two of the following: renal or liver impairment, coagulopathy, acute respiratory distress syndrome, or skin involvement [2, 3]. Epidemiological studies have shown increasing incidence of invasive GAS infections since SARS-Cov-2 lockdowns, suggesting loss of group immunity [1, 4]. Another rare disease caused by this pathogen is primary GAS peritonitis (PSAP) [5]. Primary peritonitis is defined as peritonitis without an intra-abdominal source [6, 7]. Due to its rarity, initial clinical and radiological evaluation may suspect secondary peritonitis, which results from an intra-abdominal pathology often requiring immediate surgical exploration. For PSAP, antibiotics are the primary treatment [5], although rapid onset and acute abdomen often lead to surgical exploration to exclude secondary peritonitis. This highlights the difficulty of diagnosing this condition, which can be fatal [8]. We present such a case, to raise awareness of this rare and severe condition.
Case report
A 51-year-old woman with no past medical history consulted the emergency department for severe abdominal pain within the last 12 h, associated with bilious emesis and diarrhea. She presented with fever, hypotension, and tachycardia requiring fluid resuscitation and continuous noradrenaline. Clinical exam found right hemi-abdomen pain, with guarding and rebound tenderness. Blood work demonstrated leukopenia of 3 × 109/l, normal C-reactive protein, and elevated procalcitonin (1.43 μg/l). No other sources of infection were found.
An abdominal CT scan with intravenous contrast (Fig. 1) demonstrated abdominal free fluid without pneumoperitoneum, associated with diffuse thickening of small bowel walls and extra-hepatic bile duct dilatation. All vascular mesenteric axes were permeable.
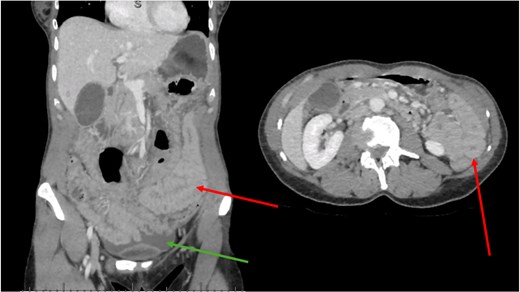
CT scan. Green arrow shows free fluid. Red arrows show diffusely thickened bowel wall.
Intravenous metronidazole and ceftriaxone were started, and the patient was transferred to the intensive care unit. Hetero-anamnesis excluded urinary tract infection, skin lesions, recent travel, or menstruation with tampon use.
Due to refractory hypotension, exploratory laparoscopy was performed, where diffuse fibrino-purulent peritonitis was found. Samples were collected for microbiological cultures. Due to the severity of her condition, conversion to laparotomy was decided to exclude a hollow viscus perforation. Upper and lower endoscopies as well as gynecological exam were also normal. The patient was kept intubated due to persistent hemodynamic instability which was complicated by multi-organ failure.
Blood cultures showed a chain Gram-positive bacteria on the first post-operative day (POD), and clindamycin was added to the antibiotic regimen. On POD 2, a GAS pyogenes was confirmed. Cultures of the abdominal fluid were sterile. The patient was extubated on POD 4, transferred to the surgical ward on POD 5, and discharged on POD 9. Follow-up 1 week after discharge was uneventful.
Discussion
Primary peritonitis is rare, accounting for 1% of all cases of peritonitis [6], and is distinguished from secondary peritonitis by the lack of an intra-abdominal causative agent. Primary peritonitis most commonly affects patients with immunosuppression, chronic liver, or kidney diseases [7] and is therefore extremely rare in patients with no comorbidities.
GAS infections predominantly affect the oropharynx, lung, and cutaneous tissues, but can present as a broad spectrum of clinical severity in case of Toxic Shock Syndrome. In our case report, the patient suffered from PSAP with Toxic Shock Syndrome. PSAP most commonly affects otherwise healthy individuals and presents with a fulminant progression within hours [5]. Due to the rarity of this disease, the majority of literature consists of case reports or small case series. A systematic review by Westwood and Roberts [5], including 23 articles and 32 patients, found that the median age of diagnosis was 38 years old, with a male to female ratio of 1:4. Another review by Malota et al. [9] including 26 publications and 35 patients found only eight patients (23%) had risk factors for primary peritonitis such as immunosuppression, liver cirrhosis, or diabetes. Initial source of the streptococcus may be from airborne transmission from close contacts, or of vaginal, cutaneous, or oropharyngeal origin; although in the majority of cases there is no primary source found [5, 10], as in this patient’s case. The pathogenesis of this disease is therefore controversial, as Group A streptococcus are not commonly part of the usual enteric flora [11]. The pathogenesis of primary peritonitis is also not clear, with hypothesizes such as increased translocation of intestinal bacteria, retrograde inoculation from the genitourinary tract, or hematogenous routes having been discussed [9].
This case highlights the difficulty of diagnosing this clinical entity, as blood cultures take time to develop, and therefore primary streptococcal peritonitis is a diagnosis of exclusion until cultures can prove the presence of the pathogen. In the setting of our patient, with worsening septic shock and an acute abdomen, surgical exploration was mandatory to exclude secondary peritonitis. This trend was confirmed in several studies, with about 85% of patients undergoing surgical exploration [5, 10]. Whether subsequent peritoneal lavage helps diminish the bacterial load and may contribute to favorable outcomes is still debated [9, 10], no comparative studies having been performed for this rare diagnosis. Studies have shown that this is of no use in cirrhotic patients with spontaneous bacterial peritonitis [12], which could be an argument against any benefit of peritoneal lavage.
Management of PSAP therefore requires a high level of suspicion. With the current increasing rate of streptococcal infections, physicians should be aware of this rare condition. Patient history and clinical exam should focus on locating a primary source, such as an oropharyngeal, cutaneous, or gynecological infection. Prompt blood and primary source cultures should be drawn, followed by broad-spectrum antibiotics and fluid resuscitation when necessary. Chosen antibiotics should ensure adequate coverage of Gram-positive bacteria, with the adjunct of Clindamycin in case of Toxic Shock Syndrome due to its toxin suppressant properties [2, 13]. In this patient’s case, the addition of clindamycin at POD 1 was probably a key factor in her favorable outcome.
Conclusion
PSAP in healthy patients is rare and requires a high level of suspicion to avoid surgical exploration. Blood cultures and suspected primary site cultures should promptly be performed, followed by antibiotic therapy. Surgery is avoidable but can exclude secondary peritonitis.
Author contributions
L.S. and S.H. are co-first authors and made equal contributions to this manuscript, with the fourth author (O.M.) being the supervising author. All authors have read and agreed to the published version of the manuscript.
Conflict of interest statement
The authors declare no conflict of interests.
Funding
This research received no external funding.
Data availability
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Institutional review board statement
Not applicable.
References
Author notes
Lukas Sommer and Sidney Heersche are co-first authors.



