-
PDF
- Split View
-
Views
-
Cite
Cite
Quan Chen, Cuiyan Yang, Hui Chen, Chuanfeng Ke, Chylothorax-induced diaphragmatic herniation: a novel complication of Gorham-Stout syndrome, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf772, https://doi.org/10.1093/jscr/rjaf772
Close - Share Icon Share
Abstract
A young male with Gorham-Stout syndrome (GSS), a rare lymphovascular disorder causing bone loss, presented with cough, difficulty breathing, and chylous pleural effusion infected with Staphylococcus aureus. Despite thoracic duct ligation, his chylothorax recurred. Imaging showed bone lesions in the sternum and ribs, along with thickened pleura. Electromyography revealed mild bilateral phrenic nerve motor conduction abnormalities. A year later, he developed a symptomatic left diaphragmatic hernia with partial bowel obstruction that required surgical repair. This case highlights the need for early diaphragm monitoring in GSS patients with chylothorax to prevent serious complications.
Introduction
Gorham-Stout syndrome (GSS) is an extremely rare disorder [1] characterized by progressive bone resorption, leading to thinning and localized disappearance of bone trabeculae [2]. These structures are replaced by hyperplastic fibrous connective tissue, proliferating capillaries, blood sinuses, and aggregates of vascular tissue [3]. The involvement of ribs or scapula led to chylothorax and were considered a poor prognostic factor [4]. We reported a case of GSS in a patient with bilateral chylothorax that was complicated by a diaphragmatic hernia.
Case report
November 2023, a male patient experienced recurrent coughing, difficulty breathing, and intermittent fever following intense physical activity. Diagnosed by external hospital as bilateral chylous pleural effusion and pneumonia. To control chylous pleural effusion and alleviate respiratory distress, the patient opted for right single-port thoracoscopic thoracic duct ligation [5], thoracoscopic adhesion release, and pleural fixation. Despite the surgery, a significant amount of pleural effusion continued to drain, impacting the patient’s daily life.
December 2023, he was admitted to our hospital for further diagnosis and treatment. The chyle test for pleural effusion was positive. Staphylococcus aureus was found in pleural effusion culture. Serum carbohydrate antigen 125 was the only elevated tumor marker (212 U/mL; reference value: 0–24 U/mL) despite a full panel being tested in both serum and pleural fluid. No malignant cells were detected in the effusion. Other laboratory parameters fell within normal ranges or demonstrated only minor deviations. A positron emission tomography-computed tomography (PET/CT) scan demonstrated generalized decreased bone density with multiple osteolytic lesions of varying sizes (Fig. 1A–D, Fig. S1). Bilateral pleural thickening was observed, showing increased fibroblast activation protein inhibitor (FAPI) metabolism without corresponding fluorodeoxyglucose (FDG) uptake (Fig. 1E and F). Electromyography revealed mild conduction slowing at the posterior borders of bilateral sternocleidomastoid muscles (left 0.3 Mv; right 0.4 Mv; reference value ≥0.7 Mv; Fig. S2). The pathological examination of the patient's right iliac bone tissue revealed progressive osteolysis (Fig. 2A), featuring sparse and slender bone trabeculae (Fig. 2B) with partial fragmentation (Fig. 2C). Together with the clinical presentation, the patient was diagnosed with GSS [6]. The patient was treated with sirolimus 2 mg once daily [7], and continued to follow a low-fat diet. Follow-up 18F-FAP-NUR PET/CT [8] scans at one year showed results consistent with the previous findings.
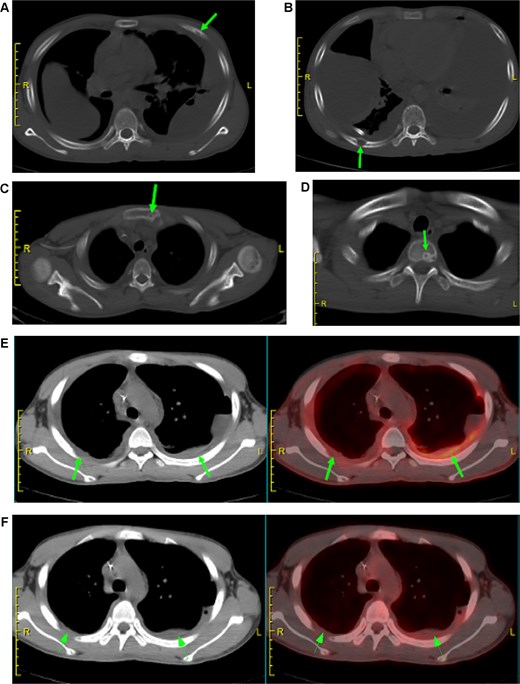
PET/CT findings of multifocal skeletal involvement and pleural abnormalities. (A–D, arrows) PET/CT scan demonstrates massive osteolytic destruction of the left 4th rib, right 8th rib, sternum, and a thoracic vertebra. (E, F, arrows) Bilateral pleural thickening with markedly increased FAPI uptake but absent FDG metabolism. Notes: (A–D) Arrows indicate sites of extensive osteolytic destruction with corresponding metabolic foci. (E) Arrow highlights FAPI-avid diffuse pleural thickening, suggestive of progressive fibrotic change. (F) Corresponding FDG-PET image shows no significant metabolic activity, emphasizing the differential diagnostic value of FAPI in this context.
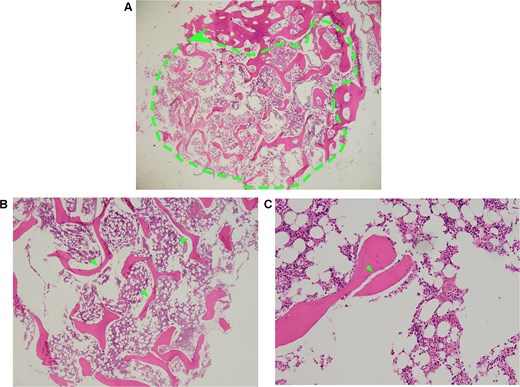
Histopathology of right iliofemoral tissue biopsied. (A, dashed outline) Low magnification shows extensive bone resorption and loss of normal bone structure. (B, arrows) high magnification displays sparse and slender bone trabeculae. (C, arrow) High magnification view highlights the area of bone microfracture. Notes: (A) Dashed outline denotes extensive osteolytic area with pathological bone loss. (B) Arrows indicate thin, attenuated, and fragmented bony trabeculae. (C) Arrow points to sites of trabecular breakdown and microstructural failure.
January 2025, on the premise of denying a history of blunt abdominal or chest trauma, the patient developed a left diaphragmatic hernia (Fig. 3A–C). Initially managed conservatively, the condition progressed (Fig. 3D and E), prompting laparoscopic diaphragmatic hernia repair to relieve the obstruction and reconstruct the diaphragm. Intraoperatively, a weakened diaphragmatic area was noted, with a ~ 6 × 5 cm defect (Fig. 4A). The right diaphragm appeared normal (Fig. 4B). The hernia sac was partially closed with continuous 3–0 sutures, leaving a 0.5 cm opening. After anesthesiologist-confirmed bilateral ventilation, the sac was fully closed with defect reinforcement using Burch sutures (Fig. 4C). Due to extensive diaphragmatic weakness and recurrence risk, we placed a synthetic mesh over the weakened area (Fig. 4D), then covered it with an anti-adhesive biologic mesh secured by sutures (Fig. 4E) to prevent adhesions. Postoperative imaging confirmed successful repair without adhesions (Fig. S3), with good patient recovery. At the one-month telephone follow-up, the patient was asymptomatic and reported no complaints.
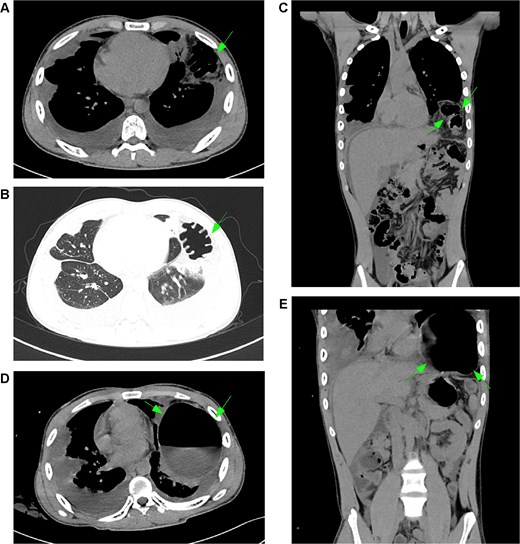
Development and progression of a left diaphragmatic hernia. (A–C, arrows) January CT scan demonstrates a newly developed left diaphragmatic hernia with herniation of abdominal contents into the thoracic cavity. (D, E, arrows) Follow-up CT scan in March shows significant progression of the herniation with increased volume of herniated content and more pronounced displacement of thoracic structures. Notes: (A–C) Arrows indicate the initial presentation of the left diaphragmatic defect, showing herniation of the omentum and bowel loops. (D, E) Arrows highlight interval progression, demonstrating substantial enlargement of the hernia sac and increased mass effect on the adjacent lung parenchyma.
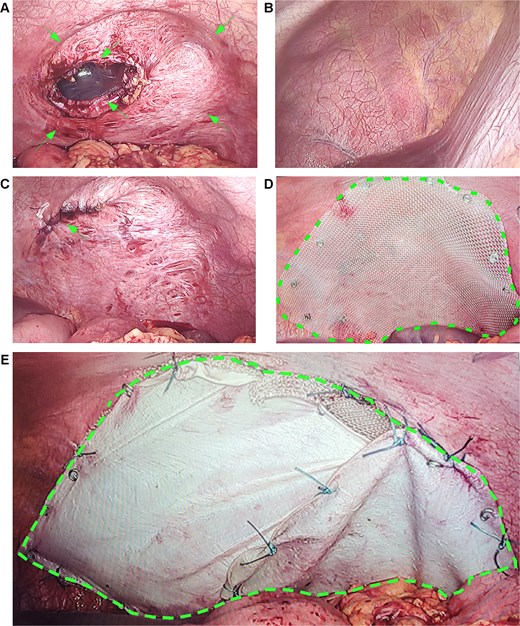
Intraoperative findings and repair of left diaphragmatic hernia. (A, arrows) The left diaphragm demonstrates a 20 × 18 cm attenuated zone and a 6 × 5 cm distinct defect. (B) The intact right diaphragm is shown for comparison. (C, arrow) Primary suture repair of the diaphragmatic defect following lung re-expansion. (D, dashed outline) Placement of a prosthetic mesh over the attenuated area for reinforcement. (E, dashed outline) A biological barrier is fixed over the mesh to prevent adhesions. Notes: (A) Arrows point to the thinned, weakened diaphragmatic area and the full-thickness defect. (C) Arrow indicates the suture line after primary closure of the hernia. (D) Dashed outline highlights the positioned mesh covering the reinforced area. (E) Dashed outline shows the overlying biological barrier that minimizes adhesion risks.
Discussion
The age of onset of GSS varies widely, with the majority of cases occurring during youth or young adulthood. Although generally considered benign, the condition can still lead to serious complications and has an unpredictable prognosis [9]. Most GSS patients initially present with respiratory distress due to chylothorax. Although a direct causal link is unproven, chylothorax may predispose patient to diaphragmatic hernia via biomechanical or pressure-mediated mechanisms.
Changes in thoracic pressure
Chylothorax-induced fluid accumulation elevates intra-thoracic pressure, restricting diaphragmatic movement. Chronic pressure dysregulation may cause diaphragmatic structural changes and weakness [10]. In this case, electromyography showed mild bilateral phrenic nerve conduction abnormalities, indicating early dysfunction.
Changes in pleural structure
Chylothorax may lead to pleural stretching or thickening [11], with such thickening potentially impairing diaphragmatic function through mechanical compression. In this patient, PET/CT demonstrated bilateral pleural thickening with elevated FAPI metabolism but minimal FDG uptake, suggesting active fibroblast proliferation and progressive pleural fibrosis.
Infection in chest cavity
Infection and chronic inflammation may lead to diaphragmatic muscle atrophy and functional impairment. The isolation of S. aureus from the chylothorax supported an infectious etiology [12], suggesting a potential cause for the subsequent diaphragmatic hernia.
Extended immobility and physical deconditioning
Patient with chylothorax and GSS often require prolonged activity restrictions, both to manage respiratory distress and to prevent skeletal complications like fractures or spinal deformities [13]. However, prolonged immobility can weaken the diaphragm and increase the risk of diaphragmatic hernia.
Nutritional deficiency
To reduce chylous fluid production, patients with chylothorax are typically placed on a low-fat diet. Impaired diaphragmatic synthesis and regeneration from malnutrition may lead to hernia [14].
GSS-associated diaphragmatic injury
Gary et al. documented lymphatic hyperplasia at the pleuro-diaphragmatic junction, resulting in diaphragmatic thinning and perforation even in the absence of hernia formation [15].
We reported the first case of chylothorax-induced diaphragmatic hernia in GSS patients. This case provides valuable surgical data and helps to fill an important knowledge gap, but several limitations warrant further discussion. The nature of a single case report precludes definitive conclusions regarding the efficacy of the mesh and surgical repair technique. Moreover, the currently limited follow-up period restricts robust assessment of potential late-term complications and disease recurrence. The patient will undergo prospective long-term surveillance with scheduled evaluations at 6 and 12 months (including exams, labs, and imaging) to collect data on durable outcomes.
Author contributions
(I) Conception and design: Q Chen, C Yang, H Chen; (II) Main surgeons: C Ke, C Yang; (III) All authors: All authors; (IV) Manuscript writing: All authors; (V) Final approval of manuscript: All authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
None declared.
Data availability
All data generated or analyzed in this study were sourced from the First Affiliated Hospital of Guangzhou Medical University.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals by any of the authors.
Consent for publication
Written informed consent was obtained from the patient and his legal representative.



