-
PDF
- Split View
-
Views
-
Cite
Cite
Thanh Tung Lai, Hideyuki Matsushima, Hisashi Kosaka, Kosuke Matsui, Gozo Kiguchi, Hidekazu Yamamoto, Takuya Ohigashi, Hoang Hai Duong, Van Khanh Nguyen, Shuji Kariya, Masaki Kaibori, Management of retrohepatic inferior vena cava injury during hepatectomy and an intravascular foreign body caused by surgical gauze migration: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf755, https://doi.org/10.1093/jscr/rjaf755
Close - Share Icon Share
Abstract
We report a case involving a hepatocellular carcinoma with massive bleeding from a large (retrohepatic inferior vena cava) RHIVC laceration during laparoscopic posterior sectionectomy, complicated by the exceedingly rare migration of surgical gauze into the left pulmonary artery (LPA). Hemostasis was achieved by manual compression and two anchoring Prolene sutures at both ends of the laceration, allowing effective RHIVC wall approximation. Given the low central venous pressure during hepatectomy, edge approximation significantly reduced bleeding and improved repair visibility. Postoperative imaging showed the gauze was lodged in the LPA, constituting an intravascular foreign body (IFB). The gauze was successfully retrieved via endovascular intervention without additional complications. Anchoring sutures with manual compression may be a helpful technique for managing a large RHIVC injury, and endovascular retrieval may provide a safe alternative to reoperation for a large IFB.
Introduction
Massive bleeding from the retrohepatic inferior vena cava (RHIVC) is one of the most serious and life-threatening complications associated with liver trauma and occurring during hepatectomy due to the difficulty in achieving hemorrhage control, especially during laparoscopic procedures [1–5]. Despite advances in surgical and perioperative techniques, injuries to the RHIVC still occur, especially involving tumors that are adherent to major vessels [2]. We report a rare case of severe hemorrhage from a large RHIVC injury that occurred during a laparoscopic posterior sectionectomy complicated by migration of a surgical gauze into the inferior vena cava (IVC) through the laceration that eventually reached the left pulmonary artery (LPA). Hemorrhage was controlled and endovascular retrieval of the gauze was achieved. This case highlights the challenges of managing RHIVC injuries and successful retrieval of an intravascular foreign body (IFB).
Case report
A 75-year-old man was referred to our institution for evaluation after the incidental finding of a liver tumor during routine surveillance. The medical history included a cerebral infarction 11 years ago, type 2 diabetes, hypertension, and percutaneous coronary intervention. He was taking aspirin daily, which was discontinued several days before surgery. The patient was asymptomatic at the time of presentation. The laboratory findings were normal and liver function was preserved (Child–Pugh A5). Dynamic contrast-enhanced computed tomography (CT) showed a solitary 53-mm lesion in segments 6 and 7, which was consistent with hepatocellular carcinoma (Fig. 1A and B). A laparoscopic posterior sectionectomy was planned.
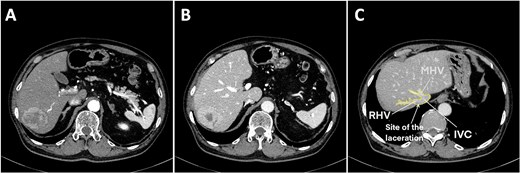
Preoperative contrast abdominal computed tomography of a hepatocellular carcinoma tumor on the posterior segment. (A) Arterial phase, (B) venous phase, and (C) right and middle hepatic vein image with the laceration site marked. Abbreviations: IVC, inferior vena cava; RHV, right hepatic vein; MHV, middle hepatic vein.
Under general anesthesia in the left lateral position, three 12-mm and three 5-mm trocars were inserted into the abdomen. The right liver was mobilized by dividing the round, right coronary, and triangular ligaments, and the inferior right hepatic veins (IRHVs) and dissection around the right hepatic vein (RHV) and IVC. Parenchymal transection was performed with intermittent Pringle maneuvers. Massive bleeding suddenly occurred from the dorsal RHV near the RHV root (Figs 1C and 2A) during transection, likely extending to the anterior RHIVC (Fig. 2B). This bleeding may have been caused by excessive traction on the liver during transection. Laparoscopic hemostasis failed and the patient developed hemorrhagic shock with the systolic blood pressure dropping to 30 mmHg. Hakuzo Sterile OP Gauze NEO (2.5 × 10 cm and 4-ply; Osaka, Japan; Fig. 3D) was used to control bleeding.
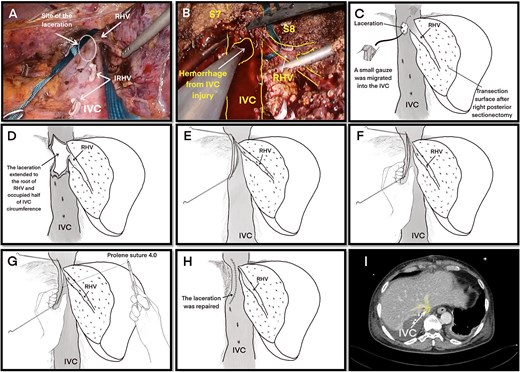
Laceration of the IVC. (A) Right liver mobilization and hanging of the RHV by laparoscopy with the laceration site marked where the small, short hepatic vein was electrocauterized. (B) Hemorrhage from the IVC injury during laparoscopic surgery. (C ) The initial laceration on the IVC was ~1 cm in size and during gauze packing a small gauze migrated into the IVC through the laceration. (D) The IVC laceration extended to the root of the RHV. The laceration was ~5 cm in length and occupied one-half of the IVC circumference. (E) The laceration was closed with two sutures at the beginning and end of the laceration. (F) Hemorrhage was minimized with finger pressure. (G) The IVC laceration was repaired with continuous sutures using 4.0 Prolene sutures. (H) The IVC injury was successfully repaired. (I) Postoperative computed tomography image showing a reduction in the IVC diameter at the hepatic vein junction by approximately one-half. Abbreviations: IVC, inferior vena cava; RHV, right hepatic vein; IRHV, inferior right hepatic vein; S7, segment 7; S8, segment 8.
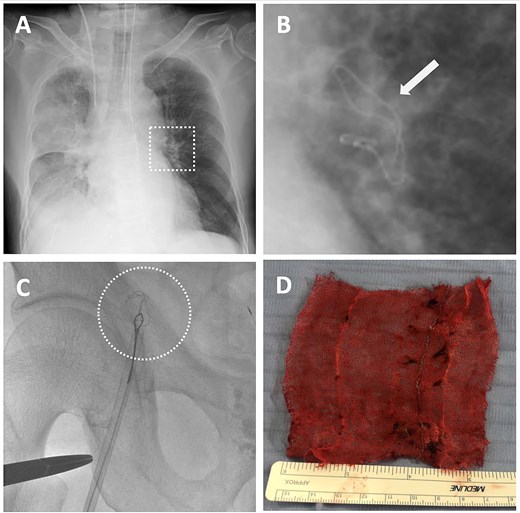
Detection of intravascular surgical gauze on radiograph and the retrieval process from the left pulmonary artery (LPA) using an endovascular technique with the right femoral vein approach. (A) X-ray image of the lung immediately after the operation. (B) The enlarged X-ray image shows the contrast of the surgical gauze at the hilum of the left lung (indicated by the arrow). (C) The snare catheter captured the surgical gauze in the LPA, passed through the right ventricle and atrium, and proceeded to the inferior vena cava and the right external iliac vein. (D) The surgical gauze was successfully retrieved through the right femoral vein.
Emergency conversion to open surgery was performed. A 0.5-cm laceration (Fig. 2C) in the anterior RHIVC had extended to a nearly 5-cm tear involving the RHV root and nearly one-half of the IVC circumference (Fig. 2D). The extended laceration was likely caused by excessive compression applied during hemostasis with residual hepatic attachment to the IVC, under which even minimal traction exacerbated the tear. Hemorrhage was initially controlled by manual compression and two anchoring Prolene sutures at both ends of the laceration (Fig. 2E). Given the low central venous pressure (~5 cmH₂O), partial closure using two anchoring sutures and manual pressure (Fig. 2F) significantly reduced the bleeding. Hemostasis was achieved with continuous 4-0 sutures (Fig. 2G). The repaired RHIVC diameter was reduced by one-half (Fig. 2H and I). Hemodynamic stability was assured and the liver transection was completed. The total blood loss was 7729 ml, the intraoperative blood transfusion volume was 3640 ml, and the operative time was 535 minutes. The gauze count revealed one missing laparoscopic gauze. Despite thorough reinspection and intraoperative chest and abdominal X-rays, the gauze was not found. Given the patient’s unstable condition, the operation was concluded and the patient was transferred to the intensive care unit.
On postoperative day (POD) 1, the patient developed hypoxemia (SpO₂ = 78%). A chest X-ray revealed a linear radiopaque shadow in the left hilar region (Fig. 3A and B). A CT confirmed the missing gauze had migrated intravascularly and was lodged in the LPA. A retrospective review of the intraoperative chest X-ray showed that the gauze had already reached that location but was missed.
Endovascular retrieval was performed 27 hours postoperatively under general anesthesia. A 12F sheath was placed via the right femoral vein. A 5F, 100-cm Headhunter catheter was advanced into the LPA guided by a 0.035-in, 260-cm Radifocus wire and then exchanged for an 8F, 90-cm guiding catheter (FUBUKI, Asahi Intecc). A 6F, 15-mm Amplatz gooseneck snare was introduced and navigated past the gauze into the distal LPA. The gauze was successfully grasped and pulled back through the LPA, cardiac chambers, IVC, and right iliac vein (Fig. 3C). Surgical exposure and venotomy of the femoral vein were performed and the gauze was removed en bloc due to difficulty passing through the 12F sheath. The procedure lasted 136 minutes. A CT showed partial thrombus in the LPA branches the following day, which resolved within 7 days. No anticoagulation was required. The patient recovered without further complications and was discharged on POD 18.
Discussion
This case exhibited several strengths. Early recognition of a life-threatening complication enabled timely multidisciplinary intervention, which was crucial for survival. Manual compression and two anchoring Prolene sutures at both ends of the IVC laceration effectively reduced bleeding and facilitated control of the hemorrhage. Complete mobilization of the right liver and IRHV dissection before parenchymal transection also controlled the bleeding. This is the second reported case in which an IFB was a surgical gauze. The benefits of interventional radiology were remarkable. Even a large object like gauze could be retrieved via endovascular intervention, which avoided a high-risk reoperation. However, this case also had two limitations. First, greater composure and caution might have prevented further IVC laceration extension. Second, earlier IFB detection could have been possible with strict adherence to the surgical counts and closer review of intraoperative imaging.
Understanding the RHIVC anatomy is crucial in this case. The RHIVC spans 8–10 cm and is extremely difficult to access because the liver is anchored to the entire length. The suprahepatic segment and the confluence of three main hepatic veins into the IVC are also remarkably short (0.5–1.5 cm). Moreover, RHIVC injury becomes more complex due to very short accessory veins from the caudate and right liver lobes (IRHV), which drain directly into the RHIVC. Hemorrhage from the RHIVC is particularly challenging to control due to limited exposure and the risk of extending lacerations during hemostasis.
The management of RHIVC injuries largely depends on the size, location, and surgical context. The standard approach begins with manual compression and/or gauze packing, followed by further exposure of the injury site, which may involve extending the incision, dividing ligaments, mobilizing the liver, and ligating the IRHV and accessory vein from the caudate lobe draining into the IVC. Repair can be performed when the laceration is identified and bleeding is under control [5]. In cases in which the injury is too extensive to manage with conventional methods, total vascular exclusion (TVE) may be required. This technique includes the Pringle maneuver, infradiaphragmatic aortic clamping, and supra- and intrahepatic IVC control. However, TVE should only be used when absolutely a sudden drop in cardiac output can occur, increasing the risk of arrhythmias or cardiac arrest. A stepwise application of TVE is recommended to minimize hemodynamic instability [2, 5, 6]. Other advanced techniques, such as the use of atriocaval (Schrock) shunt [5], balloon shunt [7], and intrahepatic balloon tamponade [8], may also be considered. However, these methods are technically demanding and require preoperative planning and equipment availability, which are often not feasible in the context of unexpected intraoperative RHIVC injuries during a hepatectomy. These approaches may be more appropriate in trauma patients, in whom bleeding is temporarily controlled and there is sufficient time to prepare the necessary devices.
This report introduced an additional technique that has proven efficacy in managing large RHIVC injuries. IVC pressure is typically maintained at <5 cmH₂O during a hepatectomy [9]. Under these conditions, placing two initial anchoring sutures at both ends of the laceration in large wounds improves wound edge approximation, significantly reduces bleeding, and simplifies the subsequent repair. Moreover, routine division of the accessory veins and IRHV during right liver mobilization is critically important. These veins are often short and fragile, making the veins highly susceptible to further tearing during RHIVC injury management. Therefore, the veins should be proactively addressed during the early stage of liver mobilization to prevent additional complications.
Port-a-Caths, guidewires, permcaths, catheters, IVC filters, stents, or sheath fragments are the most frequent IFBs, and the most frequent sites are the cardiac chambers, IVC, and pulmonary arteries [10, 11]. However, surgical gauze IFBs are exceedingly rare. The first such case was reported in 2017, in which a gauze (Trox Type A, 3 × 15 cm, Osaki Medical Co., Ltd) that entered the IVC through a middle hepatic vein root injury during a laparoscopic left hepatectomy in HCC patients migrated to the LPA and was retrieved via endovascular intervention [4]. Our case is the second such case: a gauze migrated to the IVC through the laceration, following the direction of blood flow, passed through the cardiac chambers, and reached the LPA. Endovascular retrieval was also successful in our case. However, a femoral vein incision was required for removal due to the large size of the gauze.
In conclusion, this case highlights a useful technique for managing large RHIVC injuries in which anchoring sutures at both ends of the laceration combined with manual compression can enhance hemostasis and simplify the repair. Although rare, large gauze IFBs can be safely retrieved via endovascular intervention, avoiding major reoperation and related complications.
Conflict of interest statement
None declared.
Funding
None declared.
References
- pulmonary artery
- hemorrhage
- hemostatic function
- carcinoma, hepatocellular
- foreign bodies
- hepatic resection
- lacerations
- laparoscopy
- repeat surgery
- surgical procedures, operative
- sutures
- inferior vena cava
- central venous pressure
- hemostasis procedures
- massive hemorrhage
- inferior vena cava injuries
- suture, polypropylene
- compression
- tissue approximation
- endovascular procedures
- anchoring



