-
PDF
- Split View
-
Views
-
Cite
Cite
Nithye Parvathy, Hemlata Jangir, Cherring Tandup, Nandita Kakkar, Polypoidal rectal endometriosis—a diagnostic dilemma, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf605, https://doi.org/10.1093/jscr/rjaf605
Close - Share Icon Share
Abstract
Polypoidal rectal endometriosis is a rare and diagnostically challenging manifestation of deep infiltrating endometriosis that may mimic colorectal malignancy, especially in women lacking classical cyclical symptoms. We report such a case of 37-year-old female with a 2-year history of non-cyclical rectal bleeding, weight loss, and spontaneous passage of polypoid tissue per rectum. Imaging studies revealed findings suspicious for malignancy, while repeated endoscopic biopsies showed only inflammatory or hyperplastic colonic epithelium with reactive atypia. Histopathological examination of the resected specimen rendered the diagnosis of polypoidal rectal endometriosis. Immunohistochemistry demonstrated strong estrogen receptor positivity in both glandular and stromal components. This case highlights the need to consider endometriosis in reproductive-age women with unexplained rectal symptoms. Early multidisciplinary evaluation is key to accurate diagnosis and avoiding unnecessary interventions.
Introduction
Endometriosis typified by the aberrant presence of functional endometrial glands and stroma outside the uterine cavity, affecting ~10% of reproductive-age women, with deep pelvic endometriosis occasionally extending its invasive reach to the intestinal wall. Intestinal endometriosis most frequently involves the rectum and sigmoid colon [1]. Although it’s classical, clinical manifestations encompass dysmenorrhea, chronic pelvic pain, and infertility. However, its polypoid variant poses unique diagnostic challenges and necessitating nuanced therapeutic strategies, particularly when involving the extra pelvic sites and masquerading as a rectal polyp. [2]. We report a rare and diagnostically challenging case of a 37-year-old female presenting with a 2-year history of non-cyclical rectal bleeding, significant unintentional weight loss, and spontaneous passage of polypoid tissue per rectal. Initial evaluations, including multiple biopsies, suggested hyperplastic or adenomatous rectal polyps with reactive atypia. However, a definitive diagnosis was only achieved following histopathological examination of the surgically resected specimen, which revealed polypoidal rectal endometriosis. This case highlights the diverse clinical and pathological presentations of rectal endometriosis and its potential to closely mimic malignancy, resulting in significant diagnostic uncertainty and therapeutic challenges.
Case report
A 37-year-old female presented with a 2-year history of non-cyclical, recurrent rectal bleeding, significant weight loss, altered bowel habits, and spontaneous passage of polypoid tissue per rectum. Advanced imaging modalities, including computed tomography scan and contrast-enhanced MRI, demonstrated an aggressive local disease pattern with transmural rectal wall thickening, mesorectal fat stranding, and loss of intervening fat planes, features favoring a malignant lesion (Fig. 1A and B). Despite extensive diagnostic workup, including multiple biopsies, findings remained inconclusive, limited to nonspecific granulation tissue, inflammatory changes, and hyperplastic or adenomatous polyps with low-grade dysplasia. Pelvic imaging also revealed uterine fibroids and adnexal abnormalities. Ongoing diagnostic uncertainty necessitated a lower anterior resection with diversion loop ileostomy, which was complicated by persistent postoperative purulent discharge and poor clinical recovery, Gross examination of the resected rectal segment showed a diffusely thickened wall with multiple mucosal ulcerations and a well-circumscribed polypoid lesion measuring 3 cm in maximum dimension (Fig. 1C). Histological evaluation revealed ulceration with granulation tissue, along with multiple foci of ectopic endometrial glands and stroma within the submucosa and muscularis propria. Immunohistochemistry (IHC) confirmed the endometrial origin, with strong estrogen receptor (ER) positivity in both the glands and stromal component (Fig. 2). These findings confirmed the rare diagnosis of polypoidal rectal endometriosis, illustrating the protean clinical and pathological manifestations of the disease and its potential to mimic malignancy, leading to significant diagnostic delay.
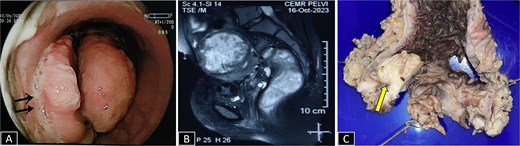
(A) Sigmoidoscopy showing a polypoidal growth with surface ulceration (arrow) in the rectum involving more than 2/3 of the circumference. (B) CE MRI pelvis in a T2-weighted sequence reveals a heterogeneously enhanced hyperintense lesion with transmural involvement reaching up to the muscularis propria. (C) Gross specimen of proctosigmoidectomy showing a pedunculated polyp with petechial hemorrhages (arrow).
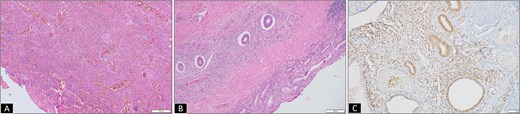
(A) Micrograph from initial rectal polyp biopsy showing complete mucosal ulceration with underlying inflammatory granulation tissue (H&E 100×). (B) Micrograph from resection specimen showing colonic mucosa with ectopic endometrial glands and stroma traversing transmurally from the lamina propria to muscularis propria (H&E 200×). (C) Endometrial glands and stroma highlighted by estrogen receptor (ER, IHC 200×).
Discussion
Intestinal endometriosis is a rare, occurring in 3%–37% of females affected with endometriosis cases [1]. It often presents with nonspecific gastrointestinal symptoms such as intermittent rectal bleeding, abdominal cramping, constipation, or altered bowel habits mimicking more common colorectal pathologies, frequently leading to diagnostic challenges, particularly when cyclical patterns are absent [3, 4]. In more severe instances, the disease progression may lead to intestinal obstruction requiring emergency surgical intervention [5].
Regardless of gynecologic history, clinicians should maintain a high index of suspicion for endometriosis in patients presenting with unexplained rectal bleeding. Colonoscopy remains a cornerstone of the diagnostic workup; however, endoscopic findings are highly variable and often mimic malignancies or submucosal tumors. Reported appearances include polypoid lesions, mucosal irregularities, and submucosal masses, as described by Shi et al. and Lee et al. [6–8]. For example, one case demonstrated a broad-based polypoid rectal lesion [3], while another revealed a partially obstructive bleeding sigmoid mass [4], both raising concern for malignancy similar to our patient.
This endoscopic variability contributes to diagnostic ambiguity, often necessitates adjunctive modalities for accurate diagnosis due to the subepithelial localization of endometriotic foci. Conventional forceps biopsies often yield nondiagnostic or misleading results due to limited mucosal involvement [8]. In such cases, endoscopic ultrasound-guided fine-needle aspiration offers superior diagnostic yield by enabling targeted sampling of deeper layers, including the submucosa and muscularis propria, and assessing lesion depth and adjacent structure involvement [7–9].
Histopathological examination remains the gold standard for identification of ectopic endometrial glands and stroma, often accompanied by smooth muscle hyperplasia, fibrosis, and occasionally hemorrhagic cystic spaces [7, 9, 10]. Rarely, endometriotic lesions may undergo malignant transformation, resulting in endometrioid or clear cell carcinoma. This is confirmed histologically by the presence of malignant epithelial cells adjacent to benign endometriotic tissue, fulfilling the criteria for endometriosis-associated malignancy. Importantly, accurate histological recognition of these histological hallmarks is essential to differentiate endometriosis from other lesions such as gastrointestinal stromal tumors or colorectal carcinoma [11, 12].
IHC serves as an invaluable adjuvant in challenging cases, particularly in atypical presentations or when malignancy is suspected. Endometriotic lesions typically express ER, progesterone receptor in the glandular component, and CD10 marking endometrial stromal components, helping to confirm diagnosis and distinguish from other pathologies. Miller et al. established that ER as the most useful single to be used stain, where there was limited tissue or a resource constrained set up. It was documented that ER expressed in all cases highlighting both endometrial glandular and stromal components [1]. In our case also, ER is the only IHC used to establish the diagnosis highlighting the limited utility of immunostains.
Conclusion
Polypoidal rectal endometriosis is a rare and elusive condition, often resulting in significant diagnostic complexity when chronic inflammatory changes obscuring underlying pathology. This case highlights the need to consider endometriosis in the differential diagnosis of rectal masses in women of reproductive age, especially when standard evaluations are inconclusive. Histopathology remains essential for definitive diagnosis, and a multidisciplinary approach is crucial for effective management.
Conflict of interest statement
None declared.
Funding
None declared.



