-
PDF
- Split View
-
Views
-
Cite
Cite
YanSong Song, ChengLong Zeng, JingKun Yang, Tao Song, Giant atypical meningioma diagnosis and clinical treatment: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf560, https://doi.org/10.1093/jscr/rjaf560
Close - Share Icon Share
Abstract
Giant atypical meningioma (WHO grade 2), predominantly female-associated and rare in males. A 23-year-old male presented with episodic limb paralysis and progressive vision/olfaction decline. Magnetic resonance imaging (MRI) revealed a > 8 cm heterogeneously enhancing tumor invading the diploic space with multi-site compression. Preoperative embolization followed by subtotal resection and adjuvant gamma knife radiosurgery achieved 3-year recurrence-free survival. This case highlights: (i) exceptional rarity of young male onset, challenging epidemiological patterns and (ii) the combined strategy of embolization, subtotal resection, and radiotherapy may overcome traditional high recurrence rates in atypical meningiomas, offering insights for individualized management.
Introduction
Meningiomas are predominantly benign tumors characterized by slow growth and represent the most common intracranial benign neoplasms, with a higher incidence in females and generally favorable prognosis [1, 2]. These tumors originate primarily from arachnoid cells and most frequently occur at the cerebral convexity, parasagittal/falcine region, sphenoid wing, and posterior fossa [3–5]. Among the subtypes, giant meningiomas and atypical meningiomas are two distinct entities. The widely accepted definition of "giant" meningioma refers to tumors >5 cm in diameter [6, 7]. Atypical meningiomas, classified as WHO grade II, occupy an intermediate position between benign and malignant tumors, exhibiting aggressive features such as rapid growth and invasive behavior. Their clinical manifestations range from symptoms of elevated intracranial pressure to focal neurological deficits caused by mass effects. This report analyses and summarizes the clinical characteristics, imaging findings, and management of a pathologically confirmed giant atypical meningioma from our institution, with discussion of our observations in the context of current literature.
Case report
A 23-year-old male presented to our hospital with a 3-day history of episodic left upper limb paralysis. Upon admission, he reported progressive left lower limb motor incoordination, temporal visual field defects in the left eye, bilateral vision,deterioration, and bilateral olfactory hypesthesia over the preceding 3 months. Three days prior to admission, he experienced sudden-onset left upper limb paralysis lasting ~10 minutes before spontaneous resolution. Cranial magnetic resonance imaging (MRI) revealed a giant meningioma (>8 cm in diameter) causing extensive compression of adjacent structures, with marked heterogeneous enhancement on contrast imaging (Fig. 1). Preoperative intracranial vascular embolization was performed, followed by tumor resection via a bifrontal-parietal approach 1 day later (Fig. 2). Intraoperative findings confirmed a tumor >8 cm in diameter with cranial bone erosion (Fig. 3). Postoperatively, the patient developed transient bilateral lower limb paralysis (muscle strength grade 0) on Day 1, which resolved completely within one month. Histopathological analysis demonstrated features consistent with atypical meningioma: sheet-like growth pattern, increased nuclear-to-cytoplasmic ratio, prominent nucleoli, focal necrosis, and immunohistochemical staining showing Vimentin(+), SSTR2(+), PR(+), EMA(−), and a Ki-67 proliferation index of 5%–10%. The patient underwent gamma knife radiosurgery at 6 months and 1 year postoperatively. Three-year follow-up MRI showed no tumor recurrence or significant progression of residual lesions (Fig. 4), with significant improvement in visual acuity, hearing, and visual field deficits.
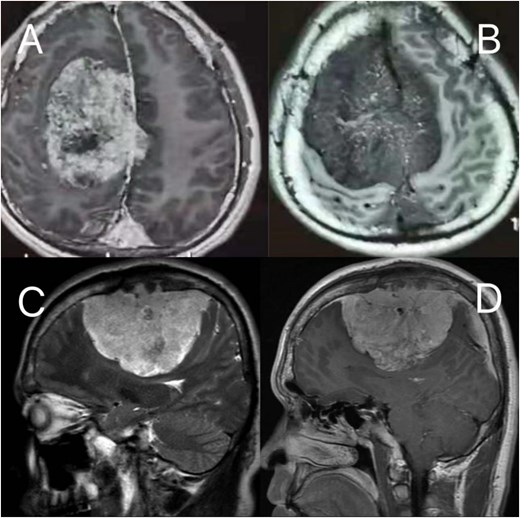
Preoperative MRI: (A, B) axial post-contrast T2-weighted and T1-weighted, respectively (C, D) sagittal position post-contrast T2-weighted and T1-weighted, respectively. The MRI findings describe a lesion in the right frontoparietal region adjacent to the superior sagittal sinus, measuring ~8.6 × 8.2 × 6.9 cm. The lesion demonstrates long T1 and T2 signal characteristics and exhibits marked heterogeneous enhancement on contrast-enhanced scans. It encircles the superior sagittal sinus and extends across the cerebral falx to the contralateral side, with multiple tortuous flow-void vascular shadows observed within and around the lesion. The mass is broadly attached to the adjacent calvarium, invading the diploic layer of the skull. Posteriorly, it extends along the superior sagittal sinus to the origin of the left transverse sinus. Compression of adjacent brain parenchyma results in irregular narrowing of the lateral ventricle and leftward shift of midline structures. Additionally, the cerebellar tonsils appear pointed and inferiorly displaced.
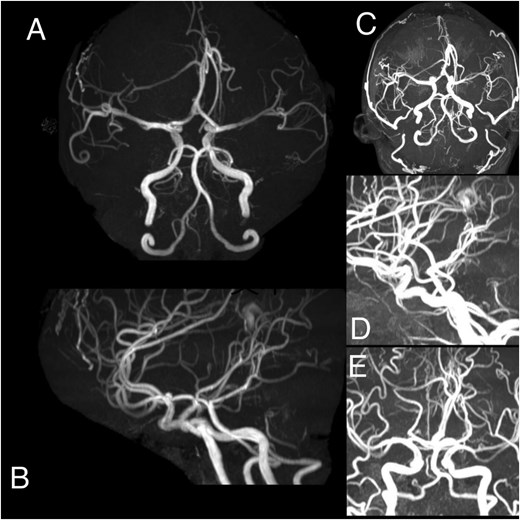
(A–E) Angiography demonstrates a hypervascular space-occupying lesion supplied by branches of the right anterior cerebral artery, right middle meningeal artery, right superficial temporal artery, left anterior cerebral artery, left middle meningeal artery, and left superficial temporal artery.
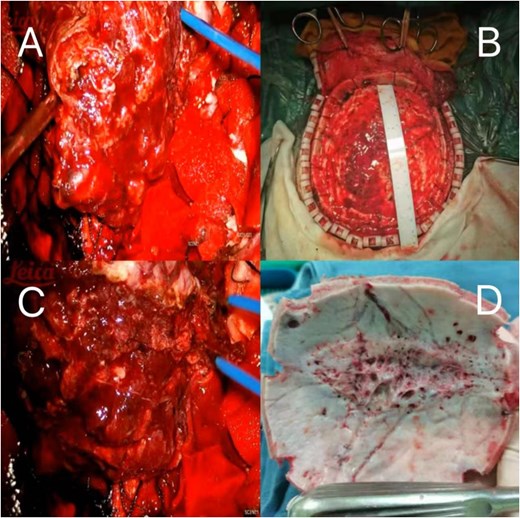
(A–C) Showing the tumor is large in size, rich in blood supply, and unclear demarcation from the surrounding tissues, occupying the entire cranial roof. (D) Extensive erosion of the skull.
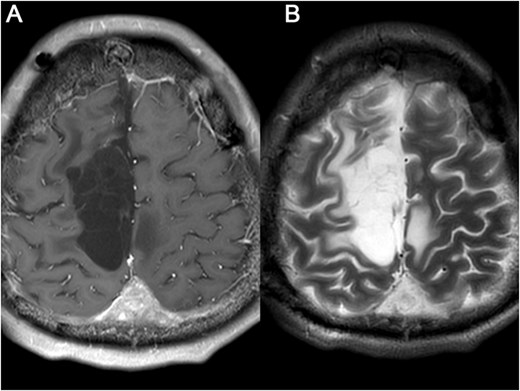
Brain MRI was re-examined after 5 months (A) and 17 months later (B), respectively, and showed that the residual tumor had not progressed and no other recurrence lesions were found.
Discussion
Meningiomas predominantly occur in middle-aged women with generally favorable prognoses, whereas atypical meningiomas and giant meningiomas are clinically rare. This case is particularly unusual due to the patient's young male demographic. Given the tumor's invasion into venous sinuses (Videos 1 and 2) and cranial bone, only subtotal resection was achievable, necessitating adjuvant radiotherapy for residual lesions. Studies confirm that postoperative gamma knife radiosurgery enhances sustained local tumor control [8].
Due to the tumor's massive size and hypervascularity, preoperative arterial embolization was performed. While preoperative embolization has been shown to reduce surgical complications and long-term disability rates, conclusive evidence supporting its efficacy in minimizing intraoperative blood loss remains limited [9–11]. Atypical meningiomas exhibit high 5-year recurrence rates of 40%–60%. However, follow-up evaluations at 1 and 3 years in this case revealed no tumor recurrence or significant progression of residual lesions, suggesting personalized treatment strategies may transcend traditional prognostic frameworks. This outcome underscores the efficacy of multimodal therapy (embolization + surgery + radiotherapy) and aligns with the hypothesis that individualized approaches could improve outcomes beyond conventional expectations. Notably, the absence of recurrence at 3-year follow-up contrasts with the typical 40%–60% recurrence rates reported in literature, further supporting the potential of tailored therapeutic regimens.
Conclusion
This case highlights the critical importance of meticulous preoperative planning, appropriate selection of surgical approaches, and rigorous postoperative follow-up in achieving favorable outcomes for rare meningioma cases. The multimodal therapeutic approach proved essential for successful management.
Conflict of interest statement
None declared.
Funding
None declared.
Consent
Patient consent was obtained.



