-
PDF
- Split View
-
Views
-
Cite
Cite
Xiabing Qin, Zhengyi Hu, Qifeng Ou, Double-paddle anterolateral thigh perforator flap for extensive soft-tissue reconstruction of the injured hands and feet, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf538, https://doi.org/10.1093/jscr/rjaf538
Close - Share Icon Share
Abstract
This study investigates the outcomes of double-paddle anterolateral thigh (ALT) perforator flaps in repairing hand and foot wounds, with emphasis on intraoperative vascular variations. Between April 2019 and April 2021, 10 cases with large defects were planned to be treated using double-paddle ALT flaps based on two descending branches of the lateral circumflex femoral artery (DB-LCFA). In three cases, one perforator arose from an atypical source, and in one case, both skin paddles traced back to a single perforator bifurcating near the skin. All flaps survived, except for one partial necrosis and one intraoperative perforator injury. Most donor sites were closed primarily; two required additional coverage. Follow-up (3–15 months) showed good functional and aesthetic recovery. These results support the double-paddle ALT flap as a viable reconstructive option. However, anatomical variations demand intraoperative adaptability, and when long pedicles are required, converting to two independent flaps may offer better reliability.
Introduction
As modern industrialization progresses, the incidence of complex soft-tissue defects in the hands and feet has significantly increased. These defects often present significant challenges due to their large size, irregular shapes, and the involvement of multiple soft-tissue regions. Simple debridement, suturing, or skin grafting is insufficient to address the extensive skin and soft-tissue loss caused by such injuries, necessitating the use of flap transplantation for wound coverage [1, 2].
Large and irregularly shaped wounds on the extremities often exceed the capacity of a single flap, leaving the donor site unable to close directly and necessitating additional skin grafting. This not only complicates the procedure but also increases patient trauma and discomfort. Alternatively, using two or more flaps requires anastomosing multiple vascular pedicles, further intensifying the surgical complexity and burden on the patient [3].
The double-paddle anterolateral thigh (ALT) perforator flap was primarily designed to enable the primary closure of the donor site while simultaneously addressing non-adjacent soft-tissue defects. Each paddle is perfused by a separate descending branch of the lateral circumflex femoral artery (DB-LCFA), each of which can be traced back to the main trunk. This configuration significantly reduces surgical time by limiting the need for microsurgical anastomosis to a single connection between the LCFA and the recipient vessels [4, 5].
Here, we present our experience with the use of double-paddle ALT flaps to repair extensive and multiple defects of the hands and feet in 10 cases. This report highlights not only the surgical outcomes but also the strategic adjustments made in response to the complexities arising from anatomical variations and the adaptability required during surgical planning.
Materials and methods
Patient details
The study retrospectively analyzed 10 cases, including 7 male and 3 female patients, aged 35 –55 years, with a mean age of 46. The injuries included six cases of mechanical trauma, two cases of crush injuries, and two cases resulting from traffic accidents. All patients presented with varying degrees of bone or tendon exposure and intrinsic muscle defects (Table 1).
| Patient . | Age (years) . | Gender . | Mechanism of injury . | Location of injury . | Dimension of defect (cm2) . | Size of flap (cm2) . | Vascular compromise . | Secondary surgeries . | Follow up (months) . | Donor site . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Female | Machine injury | Right hand | 12 × 9 | 13 × 10 | none | Fingers splitting | 3 | Healed uneventfully |
| 2 | 55 | Male | Crush injury | Right hand | 11.5 × 10 | 12 × 11 | none | none | 12 | Healed uneventfully |
| 3 | 41 | Female | Traffic injury | Left foot | 15 × 8 | 16 × 9 | none | none | 10 | Healed uneventfully |
| 4 | 48 | Male | Machine injury | Left foot | 20 × 12 | 21 × 13 | Partial necrosis | Debridement of necrosis | 11 | Covered by skin grafting |
| 5 | 40 | Male | Machine injury | Left hand | 13 × 10.5 | 14 × 11 | none | none | 15 | Healed uneventfully |
| 6 | 51 | Male | Traffic injury | Left hand | 12 × 10 | 13 × 11 | none | none | 5 | Healed uneventfully |
| 7 | 45 | Female | Crush injury | Left foot | 18 × 12 | 19 × 13 | none | none | 6 | Healed uneventfully |
| 8 | 44 | Male | Traffic injury | Right hand | 11 × 8.5 | 12 × 9 | none | Fingers splitting | 8 | Healed uneventfully |
| 9 | 45 | Male | Machine injury | Right hand | 15 × 9 | 16 × 10 | none | none | 10 | Covered by a pedicled iliac superficial perforator flap |
| 10 | 47 | Male | Machine injury | Left hand | 14 × 7 | 15 × 8 | none | none | 10 | Healed uneventfully |
| Patient . | Age (years) . | Gender . | Mechanism of injury . | Location of injury . | Dimension of defect (cm2) . | Size of flap (cm2) . | Vascular compromise . | Secondary surgeries . | Follow up (months) . | Donor site . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Female | Machine injury | Right hand | 12 × 9 | 13 × 10 | none | Fingers splitting | 3 | Healed uneventfully |
| 2 | 55 | Male | Crush injury | Right hand | 11.5 × 10 | 12 × 11 | none | none | 12 | Healed uneventfully |
| 3 | 41 | Female | Traffic injury | Left foot | 15 × 8 | 16 × 9 | none | none | 10 | Healed uneventfully |
| 4 | 48 | Male | Machine injury | Left foot | 20 × 12 | 21 × 13 | Partial necrosis | Debridement of necrosis | 11 | Covered by skin grafting |
| 5 | 40 | Male | Machine injury | Left hand | 13 × 10.5 | 14 × 11 | none | none | 15 | Healed uneventfully |
| 6 | 51 | Male | Traffic injury | Left hand | 12 × 10 | 13 × 11 | none | none | 5 | Healed uneventfully |
| 7 | 45 | Female | Crush injury | Left foot | 18 × 12 | 19 × 13 | none | none | 6 | Healed uneventfully |
| 8 | 44 | Male | Traffic injury | Right hand | 11 × 8.5 | 12 × 9 | none | Fingers splitting | 8 | Healed uneventfully |
| 9 | 45 | Male | Machine injury | Right hand | 15 × 9 | 16 × 10 | none | none | 10 | Covered by a pedicled iliac superficial perforator flap |
| 10 | 47 | Male | Machine injury | Left hand | 14 × 7 | 15 × 8 | none | none | 10 | Healed uneventfully |
| Patient . | Age (years) . | Gender . | Mechanism of injury . | Location of injury . | Dimension of defect (cm2) . | Size of flap (cm2) . | Vascular compromise . | Secondary surgeries . | Follow up (months) . | Donor site . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Female | Machine injury | Right hand | 12 × 9 | 13 × 10 | none | Fingers splitting | 3 | Healed uneventfully |
| 2 | 55 | Male | Crush injury | Right hand | 11.5 × 10 | 12 × 11 | none | none | 12 | Healed uneventfully |
| 3 | 41 | Female | Traffic injury | Left foot | 15 × 8 | 16 × 9 | none | none | 10 | Healed uneventfully |
| 4 | 48 | Male | Machine injury | Left foot | 20 × 12 | 21 × 13 | Partial necrosis | Debridement of necrosis | 11 | Covered by skin grafting |
| 5 | 40 | Male | Machine injury | Left hand | 13 × 10.5 | 14 × 11 | none | none | 15 | Healed uneventfully |
| 6 | 51 | Male | Traffic injury | Left hand | 12 × 10 | 13 × 11 | none | none | 5 | Healed uneventfully |
| 7 | 45 | Female | Crush injury | Left foot | 18 × 12 | 19 × 13 | none | none | 6 | Healed uneventfully |
| 8 | 44 | Male | Traffic injury | Right hand | 11 × 8.5 | 12 × 9 | none | Fingers splitting | 8 | Healed uneventfully |
| 9 | 45 | Male | Machine injury | Right hand | 15 × 9 | 16 × 10 | none | none | 10 | Covered by a pedicled iliac superficial perforator flap |
| 10 | 47 | Male | Machine injury | Left hand | 14 × 7 | 15 × 8 | none | none | 10 | Healed uneventfully |
| Patient . | Age (years) . | Gender . | Mechanism of injury . | Location of injury . | Dimension of defect (cm2) . | Size of flap (cm2) . | Vascular compromise . | Secondary surgeries . | Follow up (months) . | Donor site . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Female | Machine injury | Right hand | 12 × 9 | 13 × 10 | none | Fingers splitting | 3 | Healed uneventfully |
| 2 | 55 | Male | Crush injury | Right hand | 11.5 × 10 | 12 × 11 | none | none | 12 | Healed uneventfully |
| 3 | 41 | Female | Traffic injury | Left foot | 15 × 8 | 16 × 9 | none | none | 10 | Healed uneventfully |
| 4 | 48 | Male | Machine injury | Left foot | 20 × 12 | 21 × 13 | Partial necrosis | Debridement of necrosis | 11 | Covered by skin grafting |
| 5 | 40 | Male | Machine injury | Left hand | 13 × 10.5 | 14 × 11 | none | none | 15 | Healed uneventfully |
| 6 | 51 | Male | Traffic injury | Left hand | 12 × 10 | 13 × 11 | none | none | 5 | Healed uneventfully |
| 7 | 45 | Female | Crush injury | Left foot | 18 × 12 | 19 × 13 | none | none | 6 | Healed uneventfully |
| 8 | 44 | Male | Traffic injury | Right hand | 11 × 8.5 | 12 × 9 | none | Fingers splitting | 8 | Healed uneventfully |
| 9 | 45 | Male | Machine injury | Right hand | 15 × 9 | 16 × 10 | none | none | 10 | Covered by a pedicled iliac superficial perforator flap |
| 10 | 47 | Male | Machine injury | Left hand | 14 × 7 | 15 × 8 | none | none | 10 | Healed uneventfully |
Surgical procedures
Wound management included thorough debridement and hemostasis performed in the emergency department for all 10 patients. Repairs were conducted for main arteries/veins, nerves, or tendons, and fractures were reduced and stabilized. Double-paddle ALT flap was immediately harvested and applied for wound coverage in eight cases. In the remaining two cases, wounds were initially managed with vacuum sealing drainage with negative pressure irrigation for 5 to 7 days, followed by delayed reconstruction by double-paddle ALT flap.
Pre-operative flap design
For all 10 patients, preoperative planning began with the use of an ultrasound Doppler flow detector to locate and mark the perforators from the LCFA as they emerged from the fascia. The iliopatelar line—extending from the anterior superior iliac spine to the lateral edge of the patella—served as the standard reference, with a 3 cm radius circle at its midpoint designated as the primary detection area. Intraoperatively, a template matching the wound’s size and shape was fashioned to guide the flap design, ensuring that the pre-marked perforators aligned along a single longitudinal axis. For larger wounds, flap width was converted to length to reduce donor site size and facilitate direct closure, minimizing trauma and discomfort. An orderly retrograde four-sided dissection technique [6] was applied, starting with incising the lateral flap edge and dissecting toward the center while preserving perforators. After identifying the perforator exiting the fascia, a 3–5 mm incision was made around it. The vessel was dissected from superficial to deep, tracing it to the DB-LCFA, which was dissected proximally to ensure sufficient length. The remaining three sides were sequentially dissected, creating a flap pedicled on the DB-LCFA. Blood supply was verified before lobing the flap as planned and severing the pedicle once vascular supply was rechecked.
Intraoperative flap harvest and transfer
Transitioning from design to harvest, several intraoperative anatomical variations were encountered. Notably, in three cases, one of the two intended perforators originated from vascular territories other than the DB-LCFA. In one instance, a perforator arose from the transverse branch of the LCFA (TB-LCFA); by identifying its bifurcation point, a double-paddle flap was successfully designed. In another case, the bifurcation occurred on the main trunk of the LCFA; to incorporate this into the flap pedicle, the main trunk was sacrificed, yet the remaining donor sites maintained adequate perfusion without congestion after flap harvest. In a third TB-LCFA case, the pedicle was excessively long, with the TB-LCFA traced only briefly before it joined with the DB-LCFA. Consequently, two separate paddles were harvested, each supplied by an individual perforator (one from the DB-LCFA and one from the TB-LCFA), and the perforators were anastomosed prior to transfer. Additionally, in one case, both cutaneous perforators were traced to a single DB-LCFA perforator that bifurcated near its entry into the overlying skin; despite their short length, the flaps were sufficiently rotated to cover the irregular defect. Separately, intraoperative perforator damage in another case necessitated dermis removal while preserving the fascia, followed by flap rotation to complete the repair.
After harvest, the double-paddle flap was transferred to the recipient site and positioned to fully cover the wound, with temporary stabilization achieved using a few sutures. Vascular anastomosis to recipient vessels was performed under a surgical microscope, followed by reassessment of the flap’s blood supply. The wound was then closed after placement of drainage tubes, and the injured limb was secured with a temporary cast for support. The flap’s perforator was the DB-LCFA and its accompanying vein. For foot and ankle reconstruction, recipient vessels included the posterior or anterior tibial arteries and their accompanying veins, while for hand reconstruction, the radial and ulnar arteries and their accompanying veins were used.
Donor sites closure
All donor sites on the ALT were closed primarily in layers after thorough hemostasis, except in two cases—one was covered with a skin graft and the other with a translocated iliac superficial perforator flap.
Results
Of the 10 cases, 9 flaps survived completely, while 1 flap with these two skin paddles experienced partial necrosis at the distal edge of the perforator, likely due to cross-territory issues [7]. Once the necrotic margin was clearly defined, debridement was performed and residual wound was closed, leading to successful healing. None of the flaps exhibited arterial or venous complications. All flaps demonstrated good color and soft texture. The donor site requiring skin grafting healed successfully, while the donor site reconstructed with a superficial circumflex iliac artery perforator flap healed uneventfully. All remaining donor sites closed directly healed with only linear scars. Follow-up periods ranged from 3 to 15 months, with an average of 9 months (Table 1). Some flaps gained protective sensation, and functional and aesthetic recovery of the hands and feet was achieved.
Representative cases
Case 1
A 36-year-old female patient was admitted with pain and bleeding from the second to fifth fingers of the right hand for 1 hour after mechanical trauma. Emergency debridement was performed to remove necrotic and contaminated tissue (Fig. 1A and B). During the first stage of treatment, the patient underwent open reduction and internal fixation of finger fractures. The soft-tissue defect was reconstructed with a double-paddle ALT flap (Fig. 1C and D), with a superficial skin defect on index finger being covered with artificial dermis (Fig. 1E and F). Postoperatively, the flap survived without vascular complications, and the donor site healed successfully. In the second stage, internal fixation pins were removed, and skin grafting was performed for finger separation. At a 3-month follow-up, the flap exhibited good color and texture, and the right had protective sensory and satisfactory motor functions (Fig. 1G–I).
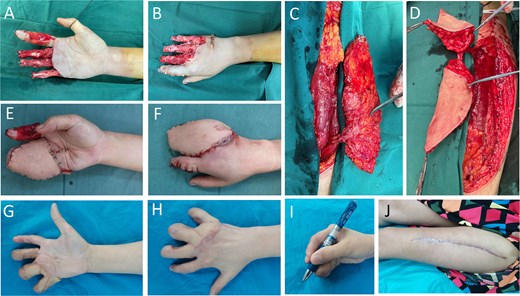
Case 1. (A and B) Crush injury to the right hand. Following debridement, the phalanges were stabilized, and a piece of artificial dermis was applied to cover the palmar surface of the index finger. Despite this intervention, significant skin- and soft- tissue defects remained on fingers 2–5 of the right hand, with underlying bone and tendon exposed. (C and D) ALT perforator flap with a cutaneous perforator emanating from DB-LCFA, splitting of flap into two paddles was based on the bifurcation near the perforating point in skin. (E and F) Right hand immediately after flap coverage. (G–I) Aesthetical and functional outcome of hand 3 months after finger-splitting surgery. (J) Donor site on thigh healed with a linear scar.
Case 2
A 58-year-old male was admitted with pain and bleeding from the first to fifth fingers of the right hand for 2 hours after mechanical trauma. The patient underwent a one-stage reconstruction procedure using a double paddle ALT flap combined with toe-nail flaps. Figure 2 illustrates the step-by-step soft-tissue reconstruction. A toe-to-thumb reconstruction was performed first, followed by coverage of the remaining wound with the double-paddle ALT flap. All three flaps demonstrated full survival. The donor site on the thigh was primarily closed and healed without complications, while the toe donor site was covered with a skin graft, which also healed uneventfully.
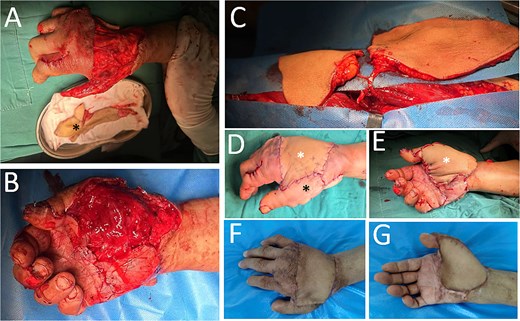
Case 2. (A) The dorsal-side defect of the right hand. The thumb was involved, and to address this, a neovascular flap from the great toe was prepared (black asterisk), ready to cover. (B) The palmar-side defect. The thumb was constructed with the toe-to-thumb flap transfer. (C) A double-paddle ALT perforator flap. (D and E) Immediately following soft-tissue reconstruction, two white asterisks—one in D and another in E—denote the two skin paddles, while a black asterisk indicates the great toe neovascular wrap-around flap. (F and G) Outcome of the reconstructed 3-month postoperatively.
Discussion
The lateral circumflex femoral artery (LCFA), originating from either the profunda femoris or femoral artery, divides into ascending, transverse, and descending branches (DB), with the descending branch being the largest and longest. This branch travels downward and laterally between the rectus femoris and vastus lateralis muscles, corresponding to the lower two-thirds of the iliac-patellar line. Along its course, it gives off muscular branches, with most (musculocutaneous perforators) passing through the vastus lateralis and fascia lata to supply the lateral thigh skin, and a smaller portion (septocutaneous perforators) piercing the fascial septum between the rectus femoris and vastus lateralis to directly nourish the skin [8]. The perforators of the descending branch, forming the ALT flap’s foundation, are relatively consistent, with an average of 4.2 cutaneous perforators in the ALT region [9] and 2–4 exceeding 0.5 mm in diameter.
Extensive studies on the LCFA perforators, combined with its large harvestable area, have established the ALT flap as a cornerstone in reconstructive surgery. The double skin paddle ALT flap, first introduced by Marsh and Chana [5], divides the ALT perforator flap into two independent paddles, each supplied by its own perforators but originate from the same vascular supply. This design allows the skin paddle to be split and combined to create an ideal shape that accommodates irregular or non-adjacent defects, enhancing versatility, enabling primary donor-site closure, preserving muscle function, and leaving a minimal linear scar for improved aesthetics [10].
In line with many other studies investigating the double paddle ALT flap [4, 11], in our study, this double-paddle configuration demonstrated exceptional adaptability, offering a wide range of rotational angles. This made it particularly effective for addressing irregularly shaped and extensive wounds on the hands and feet.
However, it is important to note that the origin, number, and location of LCFA perforators can vary significantly. While such variations may have limited impact when designing single-paddle flaps, they can pose certain challenges in the planning of double-paddle ALT flaps, as noted in the present study. Previous studies have documented these anatomical variations in the branches of the LCFA [9, 12]. In our case study, we encountered several atypical scenarios that introduced additional complexities during flap harvesting.
In our study, using the DB-LCFA double-paddle design, we identified three cases in which one of the two perforators originated from the TB-LCFA. To manage this variation, we meticulously traced the perforator to its bifurcation point—either just before or at the main trunk of the LCFA—in order to construct the double-paddle ALT flap successfully. Although this method required only a single vessel pair for anastomosis, the process of isolating the extended perforator was time-consuming, resulted in considerable muscle tissue disruption, and increased the risk of vessel kinking due to the extended pedicle length. In cases where a perforator not arising from the DB-LCFA is excessively long, it is preferable to anastomose the two perforators together once the less-dominant vessel reaches the desired length. This adjustment provides greater freedom for flap transfer while minimizing complications.
In design of double paddle ALT flap, the two cutaneous perforators can also be bifurcated from the same perforator of DB-LCFA. This anatomical variation was observed in one case (Case 1), where the bifurcation occurred within the fat-tissue layer near the perforating point in the overlying skin. Despite this, a small degree of flap rotation was achievable, enabling successful coverage of the soft-tissue defect. This technique, which utilizes vessels bifurcated from a single perforator of the DB-LCFA, has also been documented in a cohort of limb reconstruction cases [13]. Overall, this single-perforator-based, bifurcation-derived double-paddle ALT flap is less technically demanding, as it eliminates the need to trace the perforator back to the trunk of the LCFA descending branch. However, meticulous care must be taken during paddle rotation to avoid pedicle kinking or twisting.
Given this variability in the origin, number, and location of cutaneous perforators in ALT flap, preoperative designing should be assisted by ultrasound Doppler detectors [14], and meticulous intraoperative dissection is essential for double-paddle design. In cases where certain perforators follow an unusually long anatomical course, extended dissection times and unavoidable muscle damage may occur. For hand or foot reconstruction, the excessive adipose tissue in the ALT flap may necessitate debulking, which is a time-intensive process. Indeed, the intrinsic thickness of the ALT flap frequently renders it less suitable than the medial plantar artery perforator flap [15] for small or medium-sized defects. Furthermore, thumb reconstruction typically requires a toe neovascular flap for optimal outcomes, making the ALT flap a less favorable choice. In Case 2 of the present study, a wrap-around neovascular flap from the great toe [16] was employed to address the soft-tissue defect on the thumb. Subsequently, two non-adjacent soft-tissue defects—one on the palmar side and the other on the dorsum—were managed using a double-paddle ALT flap. This customized reconstruction approach achieved superior aesthetic and functional outcomes by precisely matching the flap thickness and texture to the recipient sites during flap transfer.
Conclusion
Double-paddle anterolateral femoral perforator flap is a viable solution for repairing large or multiple wounds on the hands and feet. However, anatomical variations encountered during surgery necessitate a steep learning curve and the ability to adapt intraoperatively. Thorough preoperative planning, along with precise and skilled dissection during the procedure, can minimize damage to the recipient site. In cases requiring a long pedicle, transitioning to two independent flaps with separate pedicles should be considered.
Author contributions
Xiabing Qin developed the original surgical design and wrote the draft of this manuscript. Zhengyi Hu revised the manuscript. Qifeng Ou devised the study and accomplished both the draft and finalized this manuscript. All authors read and approved the manuscript.
Conflict of interest statement
None declared.
Funding
Scientific Research Fund of Hubei Provincial Health Commission (HBJG-220065); Shiyan Science and Technology Bureau Guide Project (22Y36).
Data availability
All data generated or analysed during this study are contained within the published article.
Ethical approval
The study followed the ethical guidelines of the Ethical Committee of the Shiyan Taihe Hospital Affiliated to Hubei University of Medicine. The protocol was in accordance with the ethical standards of the Helsinki Declaration of 1975 and all subsequent revisions. Informed consent Written informed consent was obtained from the patients for their anonymized information to be published in this article.



