-
PDF
- Split View
-
Views
-
Cite
Cite
Kira C Steinkraus, Anna Nießen, Faik G Uzunoglu, Thilo Hackert, Felix Nickel, Gabriel A Plitzko, Emergent open distal pancreatosplenectomy—symptomatic giant splenic artery aneurysm: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf530, https://doi.org/10.1093/jscr/rjaf530
Close - Share Icon Share
Abstract
Splenic-artery aneurysms (SAAs) are rare but potentially life-threatening vascular lesions with a high risk of rupture, especially when symptomatic or thrombosed. Timely diagnosis and appropriate intervention are essential to prevent fatal haemorrhagic events. This case report details the management of a 60-year-old male with a giant SSA, emphasizing the necessity of urgent surgical intervention and the advantages of an open approach for rapid vascular and haemorrhage control. The patient presented with progressive upper abdominal discomfort. Computer tomography revealed a 10 × 10 cm thrombosed SAA compressing the pancreatic tail. Due to high rupture risk, open distal pancreatosplenectomy was performed. Postoperatively, the patient remained haemodynamically stable without complications or need for re-intervention. This case demonstrates the critical importance of open surgery in managing giant SAAs, offering direct vascular access and effective bleeding control. Early diagnosis and prompt surgical intervention are paramount in managing symptomatic SSA.
Introduction
Splenic-artery aneurysms (SAAs) are true dilatations involving all arterial layers and are defined by a diameter >1 cm [1, 2]. Their overall incidence is ≈0.8%, yet giant SAAs (>5 cm) are exceedingly uncommon and carry a markedly higher rupture risk, often necessitating emergent treatment [2–5]. Splenic-artery pseudoaneurysms (SAPs), produced by mural disruption and extraluminal containment, share the same catastrophic potential when large [2]. True SAAs constitute ~60% of splenic aneurysms, occur four times more often in women, and peak in the 5th–6th decades [6]. Atherosclerotic cofactors—hypertension (47%–52%), hyperlipidaemia (44%–47%), tobacco use (11%–47%)—predominate, while portal hypertension markedly elevates mortality [3, 7]. Although 80%–97.5% of SAAs are incidental findings [4, 6], symptomatic patients can report epigastric or left-upper-quadrant pain, nausea, or vomiting [6]. Spontaneous rupture, seen in 2%–10% overall and up to 28% of giant SAAs, presents with abrupt abdominal pain, possible gastrointestinal bleeding, and haemodynamic collapse [4, 8, 9]. Operative mortality ~5% electively but rises to 20% when surgery is emergent [6].
This case report presents an unusual case of a giant SAA in a 60-year-old male, highlighting the critical importance of prompt diagnosis and urgent surgical management to reduce the risk of life-threatening haemorrhagic complications.
Case presentation
A 60-year-old man was referred to the emergency department for evaluation of an incidental splenic mass seen on computer tomography (CT) the previous day, ordered for week-long, inspiration-dependent upper-abdominal discomfort. The patient had previously undergone a 5-day of cefuroxime for suspected diverticulitis, which was not confirmed in the further diagnostics.
History included a left-hip fracture (bicycle accident, 2 years prior), hypertension treated intermittently with lercanidipine, daily omeprazole, and bi-weekly testosterone injections to enhance fitness performance. He had no history of smoking, alcohol consumption, or pancreatitis and was allergic to amoxicillin. Examination showed a soft abdomen with mild left-upper-quadrant tenderness; the spleen was not palpable. Three-phase contrast CT demonstrated a 10 × 10 × 10 cm partially thrombosed, avidly enhancing lesion at the splenic hilum, displacing the pancreatic tail—features diagnostic of a SAP (Fig. 1).
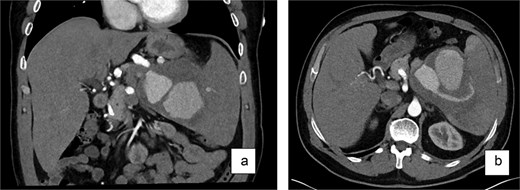
Contrast-enhanced CT scan (a—coronary view, b—axial view) of the abdomen revealed a large, partially thrombosed lesion measuring ~10 × 10 × 10 cm located at the hilum of the spleen. The lesion exhibited significant contrast enhancement in the arterial phase with peripheral thrombosis and displacement of the pancreatic tail.
A sonographic examination confirmed the presence of a vascularized mass at the splenic hilum, measuring 12.5 × 9.8 × 9.5 cm and with solid, echogenic components.
Management and surgical intervention
Because of the high rupture risk and potential exsanguination, an urgent open distal pancreatosplenectomy was performed after informed consent. A midline laparotomy provided rapid exposure and vascular control. Intraoperatively, a pulsatile giant SAA occupied the hilum and displaced the pancreatic tail. After entering the lesser sac, the proximal splenic artery was isolated along the pancreatic superior border and centrally ligated. The gastrocolic ligament and short gastric vessels were divided, the stomach retracted cranially, and the spleen mobilized en bloc with the involved pancreatic tail (Fig. 2a). The splenic artery was transected distally, and the splenic vein was ligated. The pancreas was transected after gradual compression over 3 min at the level of the mesenteric-portal axis using an Endo-GIA 60 mm stapler. No bleeding was seen from the pancreatic capsule. A ligamentum teres patch was sutured to the pancreatic stump to reduce the risk of postoperative pancreatic fistula (Fig. 2b). Two passive silicone drains were placed—one at the pancreatic transection site and one in the left lower abdomen.
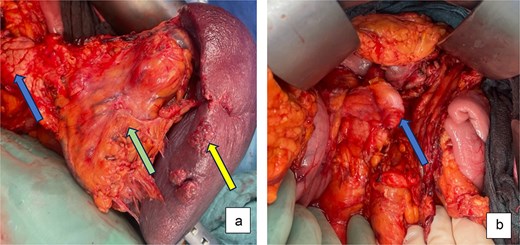
Intraoperative pictures: (a) After dissection and exposure of the giant SSA (green arrow) attached to spleen (yellow arrow) and distal pancreas (blue arrow) and (b) after distal pancreatosplenectomy with ligamentum teres patch on pancreatic stump (blue arrow).
Postoperatively the patient spent 24 h in ICU; he remained haemodynamically stable. Transferred to the surgical ward on POD 2, he recovered uneventfully. Drains were removed on POD 3 and showed no evidence of pancreatic fistula; the incision remained clean and labs evolved normally. Prophylactic anticoagulation was continued until full mobilization. He was discharged on POD 7 with thrombocytes 657 × 109/l. Histology confirmed a true SAA.
Discussion
SAAs are rare but potentially fatal, particularly when large, symptomatic, or ruptured. Endovascular and minimally invasive (MI) techniques have advanced, yet open surgery remains crucial when aneurysms are giant, unstable, or unsuitable for catheter-based therapy. In our patient, a 10-cm thrombosed SAA precluded endovascular options; an open distal pancreatosplenectomy ensured immediate, secure vascular control, and direct ligation of the splenic artery, preventing catastrophic haemorrhage.
Evidence supports this strategy. Tessier et al. showed that urgent open repair achieves excellent outcomes in unstable SAAs [9]. Conversely, for small, asymptomatic lesions, less-invasive modalities can suffice. MI approaches in elective settings minimize wound morbidity, blood loss, and recovery time [10]. In a systematic review of 107 patients, Ossola et al. reported no mortality or reoperations after MI repair, confirming safety when expertise and careful selection converge [5].
Martin et al.’s 40-year single-centre series of visceral aneurysms found fewer peri-operative complications and shorter stays after endovascular treatment, yet long-term survival matched open repair, underscoring that approach choice hinges on aneurysm anatomy and haemodynamic stability [11]. Current recommendations reflect this nuance: SAP should be treated regardless of size because of their rupture propensity, whereas true SAAs warrant intervention in women of child-bearing age, when >3 cm, enlarging, or symptomatic; tiny (<3 cm) SAAs in high-risk patients may be observed [2].
Open surgery also preserves essential training. Mastery of rapid arterial control and complex pancreatic or vascular resections is indispensable for managing life-threatening abdominal emergencies.
Thus, optimal SAA management is individualized: endovascular or MI surgery for stable, anatomically favourable lesions, and open repair for giant, ruptured, or otherwise high-risk aneurysms. Our case reaffirms that timely open intervention remains the definitive choice when rapid, direct control is required to avert fatal bleeding.
Conclusion
In summary, SAA management must be individualized. MI or endovascular techniques work best electively, particularly for small or asymptomatic lesions in stable patients. Emergencies—giant aneurysms or cases needing instant vascular control—still mandate open surgery. This approach delivers rapid, reliable haemostasis, averts life-threatening haemorrhage, and offers vital training in complex vascular and pancreatic procedures. A multidisciplinary team that blends endovascular and open surgical expertise should select the optimal strategy, maximizing outcomes in this rare, high-stakes condition. Our case underscores the need for immediate arterial control and confirms the enduring value of open surgery for high-risk SAAs.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Statement of ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- thrombosis
- abdominal pain
- aneurysm
- computers
- rupture
- splenectomy
- splenic artery
- surgical procedures, operative
- united states social security administration
- diagnosis
- splenic artery aneurysm
- vascular lesions
- vascular access
- pancreatectomy, distal
- pancreas tail
- hemorrhage control
- early diagnosis
- emergency surgical procedure
- pancreaticosplenectomy
- thrombosed aneurysm



