-
PDF
- Split View
-
Views
-
Cite
Cite
Thamer Alghamdi, Ramy Agwa, Fahad Alghamdi, Ahmed Mahmoud, Warda Othman, Different scenarios and management of complicated hepatic cystic echinococcosis: a case series, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf519, https://doi.org/10.1093/jscr/rjaf519
Close - Share Icon Share
Abstract
Cystic echinococcosis (CE) is a zoonotic illness that primarily affects the liver. Antiparasitic therapy remains the primary treatment method. Surgical intervention includes open and laparoscopic surgery, with a focus on preserving liver functionality. Data from complicated CE cases, confirmed surgically, were retrieved and presented. Six patients were included: two had a history of CE (one underwent puncture, aspiration, injection, re-aspiration [PAIR], and the other underwent right hemi-hepatectomy). Among the cases, two presented with obstructive jaundice, three had exophytic lesions, one had a large, calcified lesion, one exerted a mass effect on the intrahepatic inferior vena cava (IVC), one showed subcapsular extension, and the sixth case involved a large cyst that regressed with albendazole. In conclusion, although surgery remains the primary treatment for complicated hepatic CE, PAIR is preferred for uncomplicated cases. However, surgical challenges may be greater after PAIR.
Introduction
Human cystic echinococcosis (CE) is caused by Echinococcus granulosus, a parasite that cycle between different animal species [1, 2]. The parasite’s life cycle involves transmission from definitive to intermediate hosts through ingestion of feces contaminated with eggs from adult worms.
The primary clinical findings are mainly right upper quadrant discomfort, with two main signs, an enlarged liver or detectable mass. Acute cholangitis stands as the leading condition among CE-related complications [3]. The diagnosis of CE depends on imaging methods, with ultrasonography (US) [4]. The classification of hydatid cysts is based on their US characteristics, following established Gharbi and WHO classification systems (Table 1) [5, 6].
| Gharbi type . | Cyst morphology . | WHO Type . | Cyst morphology . |
|---|---|---|---|
| I | Homogeneously hypoechogenic cystic thin-walled lesion | CE 1 | Unilocular anechoic cyst with double line signFine internal echoes represent ‘hydatid sand’ |
| II | Septated cystic lesion | CE 2 | Cyst with multiple internal septations (multivesicular, rosette, or honeycomb appearance) |
| III | Cystic lesion with daughter lesions | CE 3A | Daughter cysts have detached laminated membranes (water lily sign) |
| CE 3B | Daughter cysts within a solid matrix | ||
| IV | Pseudo-tumor lesion | CE 4 | Cyst with heterogeneous hypoechoic and hyperechoic contents (ball of wool sign)Absence of daughter cysts |
| V | Calcified or partially calcified lesion (inactive cyst) | CE 5 | Solid and calcified wall |
| Gharbi type . | Cyst morphology . | WHO Type . | Cyst morphology . |
|---|---|---|---|
| I | Homogeneously hypoechogenic cystic thin-walled lesion | CE 1 | Unilocular anechoic cyst with double line signFine internal echoes represent ‘hydatid sand’ |
| II | Septated cystic lesion | CE 2 | Cyst with multiple internal septations (multivesicular, rosette, or honeycomb appearance) |
| III | Cystic lesion with daughter lesions | CE 3A | Daughter cysts have detached laminated membranes (water lily sign) |
| CE 3B | Daughter cysts within a solid matrix | ||
| IV | Pseudo-tumor lesion | CE 4 | Cyst with heterogeneous hypoechoic and hyperechoic contents (ball of wool sign)Absence of daughter cysts |
| V | Calcified or partially calcified lesion (inactive cyst) | CE 5 | Solid and calcified wall |
| Gharbi type . | Cyst morphology . | WHO Type . | Cyst morphology . |
|---|---|---|---|
| I | Homogeneously hypoechogenic cystic thin-walled lesion | CE 1 | Unilocular anechoic cyst with double line signFine internal echoes represent ‘hydatid sand’ |
| II | Septated cystic lesion | CE 2 | Cyst with multiple internal septations (multivesicular, rosette, or honeycomb appearance) |
| III | Cystic lesion with daughter lesions | CE 3A | Daughter cysts have detached laminated membranes (water lily sign) |
| CE 3B | Daughter cysts within a solid matrix | ||
| IV | Pseudo-tumor lesion | CE 4 | Cyst with heterogeneous hypoechoic and hyperechoic contents (ball of wool sign)Absence of daughter cysts |
| V | Calcified or partially calcified lesion (inactive cyst) | CE 5 | Solid and calcified wall |
| Gharbi type . | Cyst morphology . | WHO Type . | Cyst morphology . |
|---|---|---|---|
| I | Homogeneously hypoechogenic cystic thin-walled lesion | CE 1 | Unilocular anechoic cyst with double line signFine internal echoes represent ‘hydatid sand’ |
| II | Septated cystic lesion | CE 2 | Cyst with multiple internal septations (multivesicular, rosette, or honeycomb appearance) |
| III | Cystic lesion with daughter lesions | CE 3A | Daughter cysts have detached laminated membranes (water lily sign) |
| CE 3B | Daughter cysts within a solid matrix | ||
| IV | Pseudo-tumor lesion | CE 4 | Cyst with heterogeneous hypoechoic and hyperechoic contents (ball of wool sign)Absence of daughter cysts |
| V | Calcified or partially calcified lesion (inactive cyst) | CE 5 | Solid and calcified wall |
Although most primary CE cases present with a single cyst formation, multiple cysts are detected in 20%–40% of cases [7]. Complications associated with echinococcosis can be mechanical, superinfection or immunological (Table 2) [8].
Description of the main types of complications associated with hydatid cyst [8].
| Mechanical complications | Fistulas |
| Biliary | |
| Bronchial | |
| Plural | |
| Plural | |
| Pericardial | |
| Cutaneous | |
| Compression | |
| Displacement of adjacent structures | |
| Obstruction | |
| Portal hypertension | |
| Cyst rupture | |
| Cyst rupture | |
| Collapse middle lobe | |
| Infectious complications | Cholangitis or biliary sepsis Superinfection of CE |
| Immunoallergic complications | Urticarial angioedema Bronchial hyper-reactivity Anaphylaxis |
| Mechanical complications | Fistulas |
| Biliary | |
| Bronchial | |
| Plural | |
| Plural | |
| Pericardial | |
| Cutaneous | |
| Compression | |
| Displacement of adjacent structures | |
| Obstruction | |
| Portal hypertension | |
| Cyst rupture | |
| Cyst rupture | |
| Collapse middle lobe | |
| Infectious complications | Cholangitis or biliary sepsis Superinfection of CE |
| Immunoallergic complications | Urticarial angioedema Bronchial hyper-reactivity Anaphylaxis |
Description of the main types of complications associated with hydatid cyst [8].
| Mechanical complications | Fistulas |
| Biliary | |
| Bronchial | |
| Plural | |
| Plural | |
| Pericardial | |
| Cutaneous | |
| Compression | |
| Displacement of adjacent structures | |
| Obstruction | |
| Portal hypertension | |
| Cyst rupture | |
| Cyst rupture | |
| Collapse middle lobe | |
| Infectious complications | Cholangitis or biliary sepsis Superinfection of CE |
| Immunoallergic complications | Urticarial angioedema Bronchial hyper-reactivity Anaphylaxis |
| Mechanical complications | Fistulas |
| Biliary | |
| Bronchial | |
| Plural | |
| Plural | |
| Pericardial | |
| Cutaneous | |
| Compression | |
| Displacement of adjacent structures | |
| Obstruction | |
| Portal hypertension | |
| Cyst rupture | |
| Cyst rupture | |
| Collapse middle lobe | |
| Infectious complications | Cholangitis or biliary sepsis Superinfection of CE |
| Immunoallergic complications | Urticarial angioedema Bronchial hyper-reactivity Anaphylaxis |
CE management requires a multidisciplinary approach, incorporating surgery (with varying invasiveness), puncture, aspiration, injection, re-aspiration (PAIR), antiparasitic therapy, and monitoring [9, 10]. Traditionally, surgical resection is used as the primary treatment for hepatic CE while adding postoperative antiparasitic medication to decrease recurrence possibilities. Less invasive surgeries are currently preferred to produce fewer complications and fewer amount of tissue removal [11].
The recommended treatment for small (<5 cm) biliary system-intact Type I–III cysts is PAIR together with oral Albendazole [12]. PAIR serves as a practical choice of treatment for surgical rejection or unfit cases.
Per the American College of Gastroenterology, surgery (open or laparoscopic) is indicated for complex CE cases featuring multiple vesicles, daughter cysts, fistulas, rupture, or bacterial infection [13]. The long-term management of CE faces major hurdles because recurrences frequently appear >10 years following treatment [14].
Aim
To outline the dilemma of complicated hepatic CE clinical presentation and different management options, pointing to the need for updated management guidelines and recommendations.
Methods
We retrospectively analyzed cases of complicated hepatic CE diagnosed and treated in our hospital. All patients underwent abdominal CT, and the radiological findings later confirmed during surgery are presented and analysed in this comprehensive review. Inclusion criteria: cases of complicated hepatic CE diagnosed and treated in our hospital over the last 5 years. Cases with simple, non-complicated CE were not included.
Results and cases description
Patient characteristics for complicated hepatic echinococcosis are summarized in Table 3. Six patients were included (four females and two males), including two with a history of hepatic echinococcosis. Of these, one had previously undergone PAIR and the other had a right hemi-hepatectomy elsewhere. All patients presented with abdominal pain; among these, two developed obstructive jaundice with elevated serum bilirubin levels and dilated intrahepatic bile radicles (IHBRs), necessitating endoscopic retrograde cholangio-pancreatography (ERCP) with stent insertion. Complications observed included (overlapped in some cases):
Obstructive jaundice (two cases)
Exophytic lesions (three cases)
A large, calcified lesion causing obstructive jaundice (one case)
Mass effect on the intrahepatic inferior vena cava (one case)
Subcapsular cyst extension (one case)
A large medically responsive cyst (one case)
| Cases . | Age (years) . | Gender . | Past history of echinococcosis . | Clinical presentation . | Laboratory findings . | Radiological findings . | Management . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Female | No | 3 week history of abdominal pain, tenderness & fullness | – Eosinophilia– Elevated serum bilirubin– Positive IgG ELISA | Large calcified right hepatic lobe cyst. Dilated IHBRs | – Albendazole for 8 weeks– ERCP & stent insertion– Open surgical total peri-cystectomy and closure of the communication with CHD and RHD | Improvement |
| 2 | 35 | Female | No | Acute abdominal pain & tenderness | – Mild leukocytosis– Elevated serum bilirubin | Right hepatic intra-parenchymal cystic lesion with sub-capsular extension | – Laparoscopic endocystectomy | Improvement |
| 3 | 54 | Female | Yes (PAIR was done) | Abdominal pain | – Eosinophilia– Positive IgG ELISA | Cystic lesion (hydatid cyst) at the left hepatic lobe, with internal separations & small cysts, daughter cysts, the lesion seen partially exophytic from the liver & abutting the lesser curvature of the stomach | – Open surgical left lateral resection of the liver– Surgical view revealed showed hard calcified cystic lesion in liver segment 2 and 3 with sever adhesions to diaphragm. | Improvement |
| 4 | 31 | Male | No | Abdominal pain & tenderness with obstructive jaundice | – Elevated serum bilirubin– Elevated liver enzyme– Positive IgG ELISA | Cystic lesion with internal daughter cysts at the left hepatic lobe & was seen exophytic indenting the lesser curvature of the stomach | – ERCP & stent insertion– Albendazole for 8 weeks– Laparoscopic left lateral resection | Improvement |
| 5 | 53 | Female | No | Abdominal pain & tenderness | – Positive IgG ELISA | Three large cystic hepatic lesion; 2 at the right hepatic lobe & dome abutting and exerting mass effect on the intrahepatic IVC and the 3rd lesion is exophytic. | – Albendazole for 12 weeks– Laparoscopic cystectomies with removal of the covering parenchyma | Improvement |
| 6 | 55 | Male | Yes (right hemi-hepatectomy was done at another hospital) | Abdominal pain & tenderness | – Positive IgG ELISA | US: right hepatic lobe cyst. CT: right hepatic lobe cysts showing regressive course after Albendazole for 8 weeks | – Albendazole for 8 weeks | Improvement |
| Cases . | Age (years) . | Gender . | Past history of echinococcosis . | Clinical presentation . | Laboratory findings . | Radiological findings . | Management . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Female | No | 3 week history of abdominal pain, tenderness & fullness | – Eosinophilia– Elevated serum bilirubin– Positive IgG ELISA | Large calcified right hepatic lobe cyst. Dilated IHBRs | – Albendazole for 8 weeks– ERCP & stent insertion– Open surgical total peri-cystectomy and closure of the communication with CHD and RHD | Improvement |
| 2 | 35 | Female | No | Acute abdominal pain & tenderness | – Mild leukocytosis– Elevated serum bilirubin | Right hepatic intra-parenchymal cystic lesion with sub-capsular extension | – Laparoscopic endocystectomy | Improvement |
| 3 | 54 | Female | Yes (PAIR was done) | Abdominal pain | – Eosinophilia– Positive IgG ELISA | Cystic lesion (hydatid cyst) at the left hepatic lobe, with internal separations & small cysts, daughter cysts, the lesion seen partially exophytic from the liver & abutting the lesser curvature of the stomach | – Open surgical left lateral resection of the liver– Surgical view revealed showed hard calcified cystic lesion in liver segment 2 and 3 with sever adhesions to diaphragm. | Improvement |
| 4 | 31 | Male | No | Abdominal pain & tenderness with obstructive jaundice | – Elevated serum bilirubin– Elevated liver enzyme– Positive IgG ELISA | Cystic lesion with internal daughter cysts at the left hepatic lobe & was seen exophytic indenting the lesser curvature of the stomach | – ERCP & stent insertion– Albendazole for 8 weeks– Laparoscopic left lateral resection | Improvement |
| 5 | 53 | Female | No | Abdominal pain & tenderness | – Positive IgG ELISA | Three large cystic hepatic lesion; 2 at the right hepatic lobe & dome abutting and exerting mass effect on the intrahepatic IVC and the 3rd lesion is exophytic. | – Albendazole for 12 weeks– Laparoscopic cystectomies with removal of the covering parenchyma | Improvement |
| 6 | 55 | Male | Yes (right hemi-hepatectomy was done at another hospital) | Abdominal pain & tenderness | – Positive IgG ELISA | US: right hepatic lobe cyst. CT: right hepatic lobe cysts showing regressive course after Albendazole for 8 weeks | – Albendazole for 8 weeks | Improvement |
IHBRs: intra-hepatic biliary radicles, ERCP: endoscopic retrograde cholangio-pancreatography, CHD: common hepatic duct, RHD: right hepatic duct, PAIR: puncture aspiration injection and re-aspiration.
| Cases . | Age (years) . | Gender . | Past history of echinococcosis . | Clinical presentation . | Laboratory findings . | Radiological findings . | Management . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Female | No | 3 week history of abdominal pain, tenderness & fullness | – Eosinophilia– Elevated serum bilirubin– Positive IgG ELISA | Large calcified right hepatic lobe cyst. Dilated IHBRs | – Albendazole for 8 weeks– ERCP & stent insertion– Open surgical total peri-cystectomy and closure of the communication with CHD and RHD | Improvement |
| 2 | 35 | Female | No | Acute abdominal pain & tenderness | – Mild leukocytosis– Elevated serum bilirubin | Right hepatic intra-parenchymal cystic lesion with sub-capsular extension | – Laparoscopic endocystectomy | Improvement |
| 3 | 54 | Female | Yes (PAIR was done) | Abdominal pain | – Eosinophilia– Positive IgG ELISA | Cystic lesion (hydatid cyst) at the left hepatic lobe, with internal separations & small cysts, daughter cysts, the lesion seen partially exophytic from the liver & abutting the lesser curvature of the stomach | – Open surgical left lateral resection of the liver– Surgical view revealed showed hard calcified cystic lesion in liver segment 2 and 3 with sever adhesions to diaphragm. | Improvement |
| 4 | 31 | Male | No | Abdominal pain & tenderness with obstructive jaundice | – Elevated serum bilirubin– Elevated liver enzyme– Positive IgG ELISA | Cystic lesion with internal daughter cysts at the left hepatic lobe & was seen exophytic indenting the lesser curvature of the stomach | – ERCP & stent insertion– Albendazole for 8 weeks– Laparoscopic left lateral resection | Improvement |
| 5 | 53 | Female | No | Abdominal pain & tenderness | – Positive IgG ELISA | Three large cystic hepatic lesion; 2 at the right hepatic lobe & dome abutting and exerting mass effect on the intrahepatic IVC and the 3rd lesion is exophytic. | – Albendazole for 12 weeks– Laparoscopic cystectomies with removal of the covering parenchyma | Improvement |
| 6 | 55 | Male | Yes (right hemi-hepatectomy was done at another hospital) | Abdominal pain & tenderness | – Positive IgG ELISA | US: right hepatic lobe cyst. CT: right hepatic lobe cysts showing regressive course after Albendazole for 8 weeks | – Albendazole for 8 weeks | Improvement |
| Cases . | Age (years) . | Gender . | Past history of echinococcosis . | Clinical presentation . | Laboratory findings . | Radiological findings . | Management . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Female | No | 3 week history of abdominal pain, tenderness & fullness | – Eosinophilia– Elevated serum bilirubin– Positive IgG ELISA | Large calcified right hepatic lobe cyst. Dilated IHBRs | – Albendazole for 8 weeks– ERCP & stent insertion– Open surgical total peri-cystectomy and closure of the communication with CHD and RHD | Improvement |
| 2 | 35 | Female | No | Acute abdominal pain & tenderness | – Mild leukocytosis– Elevated serum bilirubin | Right hepatic intra-parenchymal cystic lesion with sub-capsular extension | – Laparoscopic endocystectomy | Improvement |
| 3 | 54 | Female | Yes (PAIR was done) | Abdominal pain | – Eosinophilia– Positive IgG ELISA | Cystic lesion (hydatid cyst) at the left hepatic lobe, with internal separations & small cysts, daughter cysts, the lesion seen partially exophytic from the liver & abutting the lesser curvature of the stomach | – Open surgical left lateral resection of the liver– Surgical view revealed showed hard calcified cystic lesion in liver segment 2 and 3 with sever adhesions to diaphragm. | Improvement |
| 4 | 31 | Male | No | Abdominal pain & tenderness with obstructive jaundice | – Elevated serum bilirubin– Elevated liver enzyme– Positive IgG ELISA | Cystic lesion with internal daughter cysts at the left hepatic lobe & was seen exophytic indenting the lesser curvature of the stomach | – ERCP & stent insertion– Albendazole for 8 weeks– Laparoscopic left lateral resection | Improvement |
| 5 | 53 | Female | No | Abdominal pain & tenderness | – Positive IgG ELISA | Three large cystic hepatic lesion; 2 at the right hepatic lobe & dome abutting and exerting mass effect on the intrahepatic IVC and the 3rd lesion is exophytic. | – Albendazole for 12 weeks– Laparoscopic cystectomies with removal of the covering parenchyma | Improvement |
| 6 | 55 | Male | Yes (right hemi-hepatectomy was done at another hospital) | Abdominal pain & tenderness | – Positive IgG ELISA | US: right hepatic lobe cyst. CT: right hepatic lobe cysts showing regressive course after Albendazole for 8 weeks | – Albendazole for 8 weeks | Improvement |
IHBRs: intra-hepatic biliary radicles, ERCP: endoscopic retrograde cholangio-pancreatography, CHD: common hepatic duct, RHD: right hepatic duct, PAIR: puncture aspiration injection and re-aspiration.
| WHO Type . | Main Characteristics . | Viability . | Treatment . |
|---|---|---|---|
| Cystic Echinococcosis 1 | Uniloculated cyst with ‘double line’ sign | Active Cyst (Viable) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) and 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 2 | Uniloculated cyst with regular, vascular ‘septation’ resembling daughter cysts | Active Cyst (Viable) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 3–6 months if cyst is small |
| Cystic Echinococcosis 3A | Continuous thin and regular membranes floating in cyst, resembling detached parasite layers | Traditional (Variable Viability) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) + 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 3 B | Predominately solid with daughter cysts | Traditional (Variable Viability) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 1–6 months if cyst is small |
| Cystic Echinococcosis 4 | Parasite membranes embedded in heterogenous, avascular solid content (‘ball of wool’ appearance) | Inactive (low or no viability) | Watch and wait |
| Cystic Echinococcosis 5 | Cyst with solid content with eggshell wall calcifications | Inactive (no viability) | Watch and wait |
| WHO Type . | Main Characteristics . | Viability . | Treatment . |
|---|---|---|---|
| Cystic Echinococcosis 1 | Uniloculated cyst with ‘double line’ sign | Active Cyst (Viable) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) and 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 2 | Uniloculated cyst with regular, vascular ‘septation’ resembling daughter cysts | Active Cyst (Viable) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 3–6 months if cyst is small |
| Cystic Echinococcosis 3A | Continuous thin and regular membranes floating in cyst, resembling detached parasite layers | Traditional (Variable Viability) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) + 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 3 B | Predominately solid with daughter cysts | Traditional (Variable Viability) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 1–6 months if cyst is small |
| Cystic Echinococcosis 4 | Parasite membranes embedded in heterogenous, avascular solid content (‘ball of wool’ appearance) | Inactive (low or no viability) | Watch and wait |
| Cystic Echinococcosis 5 | Cyst with solid content with eggshell wall calcifications | Inactive (no viability) | Watch and wait |
| WHO Type . | Main Characteristics . | Viability . | Treatment . |
|---|---|---|---|
| Cystic Echinococcosis 1 | Uniloculated cyst with ‘double line’ sign | Active Cyst (Viable) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) and 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 2 | Uniloculated cyst with regular, vascular ‘septation’ resembling daughter cysts | Active Cyst (Viable) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 3–6 months if cyst is small |
| Cystic Echinococcosis 3A | Continuous thin and regular membranes floating in cyst, resembling detached parasite layers | Traditional (Variable Viability) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) + 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 3 B | Predominately solid with daughter cysts | Traditional (Variable Viability) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 1–6 months if cyst is small |
| Cystic Echinococcosis 4 | Parasite membranes embedded in heterogenous, avascular solid content (‘ball of wool’ appearance) | Inactive (low or no viability) | Watch and wait |
| Cystic Echinococcosis 5 | Cyst with solid content with eggshell wall calcifications | Inactive (no viability) | Watch and wait |
| WHO Type . | Main Characteristics . | Viability . | Treatment . |
|---|---|---|---|
| Cystic Echinococcosis 1 | Uniloculated cyst with ‘double line’ sign | Active Cyst (Viable) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) and 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 2 | Uniloculated cyst with regular, vascular ‘septation’ resembling daughter cysts | Active Cyst (Viable) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 3–6 months if cyst is small |
| Cystic Echinococcosis 3A | Continuous thin and regular membranes floating in cyst, resembling detached parasite layers | Traditional (Variable Viability) | ABZ 3–6 months (small size <5 cm) Or percutaneous treatment (PAIR) + 1–6 months ABZ (Cyst 5–10 cm) Or surgery + 1–3 months ABZ (Cyst >10 cm |
| Cystic Echinococcosis 3 B | Predominately solid with daughter cysts | Traditional (Variable Viability) | Surgery + 1–3 months ABZ or modified percutaneous treatment + 1–3 months ABZ or ABZ alone 1–6 months if cyst is small |
| Cystic Echinococcosis 4 | Parasite membranes embedded in heterogenous, avascular solid content (‘ball of wool’ appearance) | Inactive (low or no viability) | Watch and wait |
| Cystic Echinococcosis 5 | Cyst with solid content with eggshell wall calcifications | Inactive (no viability) | Watch and wait |
Albendazole therapy was administered in four cases, with one cyst demonstrating regression that avoided the need for surgery. Among the five surgically managed cases, two underwent open surgery and three had laparoscopic procedures, all achieving postoperative recovery (Table 3).
Case 1
A 42-year-old woman experiences 3 weeks of right upper quadrant pain. On examination showed mild epigastric enlargement and tenderness. Laboratory tests showed eosinophilia, increases in total and direct bilirubin levels. The US discovered a large heterogenous mass with minimal expansion of IHBRs. Abdominal CT revealed large calcified right hepatic lobe cyst with dilated IHBRs (Fig. 1).
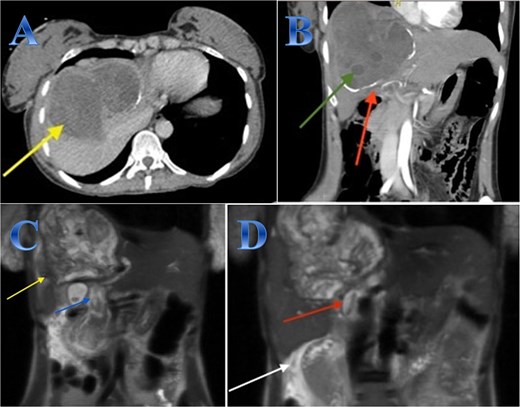
Case 1. (A & B) Sagittal and coronal CT cuts of the abdomen at venous phase showing a large peripherally calcified heterogeneous hypodense mass in the right hepatic lobe (photo A), with internal septations and small cysts (daughter cysts), with dilated IHBRs (photo B). (C & D) Coronal MRI images of the abdomen (follow up study) showing biliary extension of the lesion, CBD and the hepatic duct and sub hepatic fluid signal.
A preoperative Albendazole of 400 mg twice daily was started for 8 weeks. An ERCP paired with sphincterotomy, and stenting of the biliary ducts revealed a gelatinous substance with many daughter cysts that came through the ampulla of Vater. The severity of the lesions required an open surgical approach as it is a sizeable CE communicating with common and right hepatic ducts, treated by total peri-cystectomy and closure of the communication with common hepatic duct (CHD) and right hepatic duct (RHD). The cyst was isolated using gauze pads soaked in hypertonic saline. The cyst was punctured with a needle, and its contents were aspirated to reduce intra-cystic pressure. The gall bladder was adherent to the cyst, so cholecystectomy was done; after that, through the cystic duct, contrast material was injected, and it came through two intraparenchymal openings. These two intra-parenchymal openings were closed through deep sutures in two layers. A cholangiogram was done to make sure there was no stenosis or leakage (Fig. 1).
Case 2
A 35-year-old lady with developed right upper quadrant abdominal pain suddenly. The laboratory tests showed a mild elevation of white blood cells and bilirubin levels. Abdominal US revealed a cystic structure extending from the right to the left hepatic lobes measuring 14 × 5 cm. CT with contrast revealed right hepatic intra-parenchymal cystic lesion with sub-capsular extension (Fig. 2).
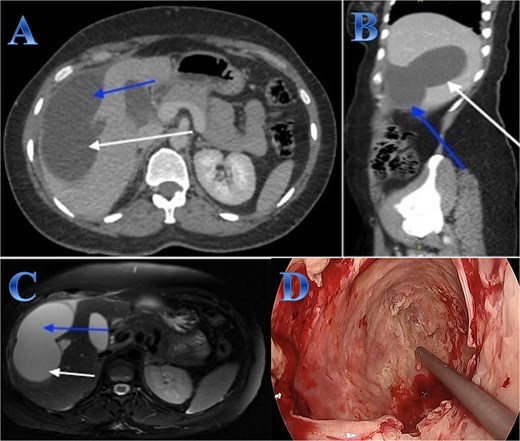
Case 2. (A & B) Sagittal and axial CT cuts of the abdomen at venous phase showing right hepatic intra-parenchymal cystic lesion with subcapsular extension with no obvious post contrast enhancement. (C) MRI images showing right hepatic intra-parenchymal cystic lesion with subcapsular extension, proven to be sealed perforation. (D) The intraoperative finding after laparoscopic endocystectomy and the area of the cyst which was occupying the right hemi-liver under the ribs and reaching the diaphragm.
Laparoscopic endocystectomy was recommended. The liver was explored, and the cyst was isolated using gauze soaked in hypertonic saline. A suction device was used to puncture the cyst and aspirate the cyst fluid. Hypertonic saline was injected into the cyst (Fig. 2).
Case 3
A 54-year-old woman presented with upper abdominal pain with a history of PAIR procedure before 5 years. The abdominal CT scan with contrast highlights left hepatic lobe calcified exophytic CE (Fig. 3).
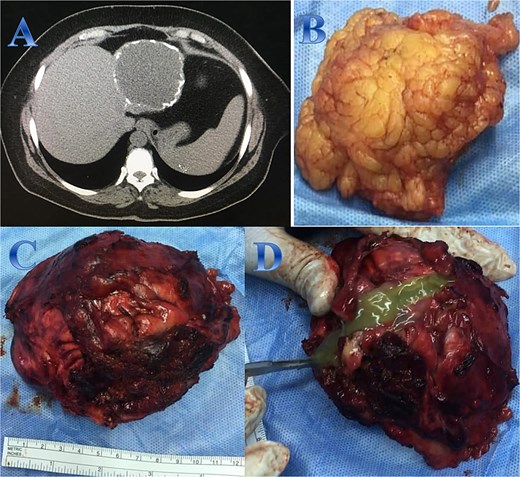
Case 3. (A) Coronal CT cut of the abdomen at venous phase showing a well-defined cystic lesion (hydatid cyst) seen at the left hepatic lobe, showing calcifications, the lesion seen partially exophytic from the liver and abutting the lesser curvature of the stomach. (B & C) The hydatid cysts that were resected from the liver (C) and from the greater Omentum (B). (D) After resection the liver hydatid cyst was opened to examine the content of the cyst which is showing jelly like structure.
For a left lateral hepatic resection, a subcostal incision allowed exploration of the calcified cystic mass, which affected segments 2 and 3 of the liver and was significantly adhered to the diaphragm and stomach. A left lateral segment (segments 2 and 3) mobilization procedure required dividing the falciform, left triangular, and left coronary ligaments. A harmonic scalpel, in combination with bipolar coagulation, completed the resection of the recurrent hydatid cyst without damaging its internal contents (Fig. 3).
Case 4
A 31-year-old male presented with a 5-days history of epigastric pain accompanied by jaundice, darkened urine, together with a clay stool. On examination, there was jaundice with epigastric tenderness. Clinical tests showed increased total and direct bilirubin levels. CT showed left hepatic lobe exophytic CE (Fig. 4).
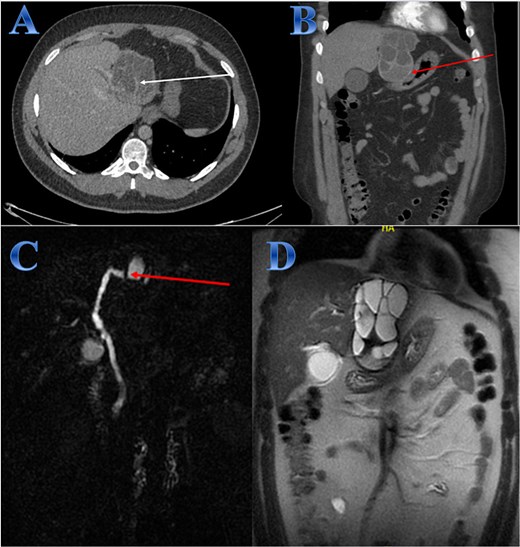
Case 4. (A & B) Post-contrast CT cuts showing well-defined cystic lesion showing internal daughter cysts noted at the left hepatic lobe and seen exophytic indenting the lesser curvature of the stomach, septate lesion is seen in the left lobe of the liver (photo A). The lesion is abutting the lesser curvature of the stomach with intact fat planes between the two (photo A). (C & D) MRI cuts showing well-defined, septate lesion, abutting the lesser curvature of the stomach. The lesion makes some mass effect off the left hepatic biliary radicles communicating with the lesion (photo C).
The patient underwent ERCP for biliary drainage. Subsequent follow-up revealed improved jaundice and reduced pain; at that time, the patient was started on albendazole, which continued for 2 months. The patient was admitted for surgery, and laparoscopic left lateral resection was done. After liver mobilization was done by detaching the falciform, left triangular and coronary ligaments to mobilize the left liver lobe. Retraction of the left lateral segment was performed to expose segments 2 and 3 which were isolated using laparoscopic gauze soaked in hypertonic saline A harmonic scalpel was used for parenchyma transection, and the left bile duct was identified between segments 4 and 2 and 3, which was transected using endovascular gastrointestinal stabler (endo-GIA). Before ending the parenchyma dissection upward, the left hepatic vein was identified and divided using endo-GIA (Fig. 4).
Case 5
A 53-year-old female presented with epigastric dull aching pain, with nausea and mild epigastric tenderness. Abdominal CT described three large hepatic CE with mass effect on the intrahepatic IVC (Fig. 5).
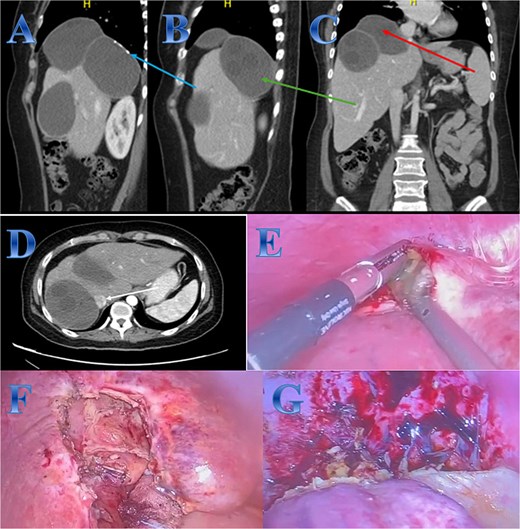
Case 5. (A, B, C & D) Three large hepatic cystic lesions seen, the largest two lesions seen at the hepatic dome and right hepatic lobe seen non-separable from each other, showing peripheral coarse calcifications (photo A), the lesions seen abutting and exerting mass effect on the intrahepatic IVC (photo D). One lesion seen exophytic superiorly to the subdiaphragmatic region elevating the right hemidiaphragm (photo C), the second lesion exophytic inferior posteriorly (photo B). (E) The content of the cyst in liver segment 8 after opening the cyst and aspiration. (F) Area of liver segment 4 after complete resection of the cyst after cholecystectomy. (G) Area above tow cysts in liver segment 7 & 8 after complete separation from diaphragm and resection of the cysts.
The patient received albendazole treatment with clinical improvement. Three months after treatment, laparoscopic peri-cystectomies were done with the removal of the covering parenchyma. During exploration, there were three large cysts; the first one was in liver segment 4a, extending to liver segment 8, compressing the intrahepatic IVC; the second one was adjacent to the first one, extending from liver segment 8 to segment 7 and the third one was in liver segment 4b extending to segment 5 and adjacent to the gallbladder. The liver mobilization was done by detaching the right triangular ligament and coronary ligament, which were fixed to the upper two cysts’ upper border, to mobilize the right liver lobe, then cholecystectomy was done. A suction device was used to puncture the cyst and aspirate the cyst fluid. The pericyst is carefully opened; the germinal layer and hydatid sand are removed using laparoscopic forceps and suction devices (Fig. 5).
Case 6
A 55-year-old male with a history of right hemi-hepatectomy secondary to a liver CE occupying the right hemi-liver; he presented with a 2-week history of epigastric and right upper quadrant pain radiating to the right shoulder pain with mild epigastric tenderness. An abdominal US revealed a cyst in the right lobe, no dilated IHBRs. The patient was started on albendazole and reevaluated again after 2 months with a CT scan. Abdominal CT showed a regressive course of the previously reported cystic lesion, seen as a well-defined cystic lesion at the right hepatic lobe measuring 6 × 4.4 × 5 cm while previously measuring 8.8 × 6.5 × 8.2 cm at AP, transverse, and vertical dimensions, respectively (Fig. 6).
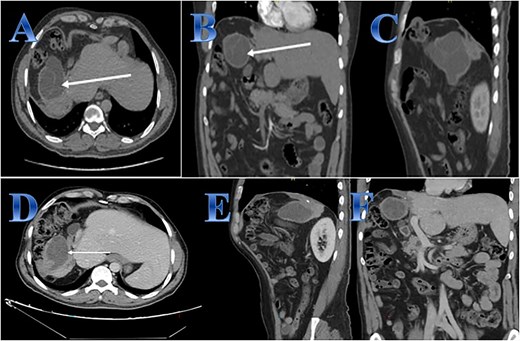
Case 6. (A, B & C) Before treatment; residual small part of right hepatic lobe is seen with irregular borders and calcifications (post-operative changes) evidence of well-defined cystic lesion seen related to the anatomical site of the resected right hepatic lobe with internal septations that showed faint septal post contrast enhancement. (D, E, F) After treatment; regressive course of previously reported cystic lesion. Interval regressive course of the previously seen well defined cystic lesion seen at right hepatic lobe measuring 6 × 4.4 × 5 cm while previously measuring 88 × 65 × 82 mm at AP, transverse, and vertical dimensions respectively.
Discussion
In endemic regions globally, increasing concerns exist regarding the growing public health burden of human CE [15]. These cysts lead to infections while producing jaundice or cholangitis after existing in the bile duct pathway and creating potential risks of anaphylaxis [16]. Particularly vulnerable to peritoneal rupture are large, superficial, thin-walled hepatic CE cysts located on the inferior or anterior hepatic surfaces.
Most patients demonstrate advanced disease stages when they finally visit medical facilities because their symptoms have progressed to complications. Those under follow-up care after treatment of infected patients need regular screenings along with their close family members. More accurate detection of hepatic hydatid cysts needs additional serological tests and biomarker assessments [17]. CT imaging is the preferred method for diagnosing complications because treatment plans are established from these results according to the case observations. Evaluating hepatic CE contains multiple conditions that share similar imaging findings with simple liver cysts, solitary bile duct cysts, hepatobiliary cystadenoma, hemangioma, and hepatic abscess [18, 19].
The world is actively pursuing the development of universal treatment procedures for CE. WHO stands by four different treatment plans that its experts use to classify cyst stages according to their recommendations [20]. According to WHO standards, the current revision of pathognomonic characteristics for CE cysts in the United States appears in table [4].
The classification system helps separating active from transitionally active and dormant cysts by assessing cyst size and shape while considering their anatomical position [21, 22]. There is no standard surgical procedure for even simple cases involving CE. Several treatment approaches exist for complex cases, including cyst ruptures and biliary fistula persistence. Multiple strategies become necessary for each case because of its complexities.
The main surgical goals for managing cysts include turning off the cyst while stopping leakage during operation, eliminating active cystic tissues, treating leftover cavities by draining them and performing omentoplasty to prevent abscess formation. Approaches for cyst treatment involve peri-cystectomy or liver resection as aggressive methods and simple drainage or cavity obliteration as more conservative treatment options. The surgical targets need execution through open conventional surgery or laparoscopic minimally invasive procedures. Minimal access surgery remained unavailable as an option because of extreme disease progression in most of our cases. Successful liver resection requires clear visualization of major hepatic vasculature and comprehensive understanding of liver segmental anatomy, as these factors significantly reduce surgical risks [23].
The study involved giving albendazole treatment to patients both before and after. The immediate surgical requirements forced the exclusion of preoperative treatment when patients presented with urgent and complicated cases, yet CT examinations after surgery demonstrated no signs of hepatic recurrence. The traditional history of unsuccessful non-surgical treatment approaches now draws renewed interest because of developing antiparasitic therapy methods. According to evidence, combining praziquantel with albendazole produces a synergistic effect that improves therapeutic outcomes [24].
ERCP represents the clinical standard procedure for diagnosing and treating cysto-biliary connections. ERCP may be required after surgery to identify and treat developing bile leaks. Patients with jaundice must undergo preoperative ERCP to reduce the likelihood of biliary leakage during surgery [25]. The two patients who received preoperative ERCP procedures experienced smaller cyst dimensions with better bilirubin measurements.
Preoperative and postoperative Albendazole treatment was provided to most patients, yet patients needing urgent surgery received a cut-short preoperative treatment period. CT imaging after the operation showed no recurrence of hepatic cysts in this patient group. Complex hepatic cysts that connect to the biliary tree while exceeding 15 cm in diameter or causing major vascular structure compression or both liver lobe involvements should be treated as complex cysts that need surgical intervention. Laparoscopic treatment of hepatic CE should be performed for simple cysts that are small and easily accessible.
Conclusions
Effective management of CE requires a multidisciplinary team approach. In our study, emergency surgery was indicated in cases presenting with peritoneal rupture. Given the risk of recurrence, CE patients require continuous postoperative monitoring. Current treatment paradigms recommend PAIR for uncomplicated hepatic hydatid disease or surgical intervention as the gold standard for complicated cases. The role of PAIR in complicated hepatic CE remains controversial, as most such cases ultimately require surgical management. Notably, prior PAIR may increase surgical complexity due to cyst rupture or adhesion formation, complicating subsequent procedures.
Author contributions
T.A. contributed to conceptualization. R.A. and W.O. were involved in data curation. R.A. made formal analysis. A.M. and F.A. made investigation. W.O. and F.A. contributed to methodology. R.A. contributed to software. T.A. made validation. A.M. contributed to visualization. W.O. and R.A. performed. Writing—review & editing and Writing—original draft.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval and/or Institutional Review Board (IRB) approval
Ethical approval for this study protocol and data publication was obtained from the Institutional Review Board of Al Baha University, Faculty of Medicine (approval No. IRB/SUR/BU-FM/2024/162).
References
WHO/OIE. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. World Health Organization and World Organisation for Animal Health, 2001. 280 pp. Available online: https://www.who.int/publications-detail-redirect/929044522X (5 November 2024, date last accessed).
Knipe H, Sattar A. Gharbi classification of cystic echinococcosis. Radiopaedia.org 2023; [online] Available at: https://radiopaedia.org/articles/gharbi-classification-of-cystic-echinococcosis-199855 (15 February 2025, date last accessed).
Venkatesh M, Knipe H, Deng F, et al. . WHO-IWGE classification of cystic echinococcosis. Radiopaedia.org 2024. Available at: https://radiopaedia.org/articles/who-iwge-classification-of-cystic-echinococcosis (15 February 2025, date last accessed).
Pascal G, Azoulay D, Belghiti J, Laurent A. Hydatid disease of the liver. In: Jarnagin WR, Allen PJ, Chapman WC, et al. (eds).
Ran B, Aji T, Jiang T, et al. . Differentiation between hepatic cystic echinococcosis types 1 and simple hepatic cysts: a retrospective analysis.



