-
PDF
- Split View
-
Views
-
Cite
Cite
Gerard P Sexton, Katherine Griffin, Rachel Lockhart, Liam Skinner, Laryngeal stenosis as a late complication of plasma cell mucositis of the larynx, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf512, https://doi.org/10.1093/jscr/rjaf512
Close - Share Icon Share
Abstract
Plasma cell mucositis (PCM) is a rare inflammatory condition of the upper aerodigestive tract with a heterogeneous clinical course. Predominately seen in the oral cavity, laryngeal involvement and the requirement for tracheostomy have been rarely described. We describe the case of a man diagnosed with airway-threatening PCM isolated to the larynx requiring a tracheostomy. He responded to oral glucocorticoids and was decannulated only to present 3 years later with severe laryngeal stenosis requiring repeat tracheostomy insertion. Medical management in the form of immunosuppression is the mainstay of treatment for plasma cell mucositis. Surgical management is necessary in a significant proportion of cases with laryngeal involvement.
Introduction
Plasma cell mucositis (PCM) is a chronic, non-neoplastic, inflammatory disorder of unknown aetiology defined by submucosal plasma cell infiltrates and epithelial hyperplasia [1]. The clinical presentation can involve any mucosal site, though the oral cavity is the most common [2]. The clinical course varies based on the location and degree of involved mucosa [3]. The natural history is unclear, with the literature base entirely composed of case series and reports [4].
A recent review found 23 cases of laryngeal PCM [5]. Isolated laryngeal involvement has been reported in seven cases [5–11]. Tracheostomy has been described in four cases, two of whom were successfully decannulated [9, 12]. There remains a paucity of long-term data regarding the prevalence of late complications to guide the need for long-term medical management. We describe an acute presentation of PCM with later progression to laryngeal stenosis requiring emergent tracheostomy.
Case report
A 48-year-old Irish man presented in acute respiratory distress to the emergency department (ED). He described worsening stridor, shortness of breath, neck tightness, and voice change over the preceding days. Past medical history was notable for gout, gastro-oesophageal reflux disease, laparotomy for acute bowel ischaemia, two incisional hernia repairs, and appendicectomy. He was an ex-smoker of 20 cigarettes/day for 10 years and took minimal alcohol.
On clinical examination, harsh stridor with increased work of breathing was noted. Flexible nasoendoscopy demonstrated generalized supraglottic oedema with impending upper airway obstruction. Intravenous dexamethasone was commenced. A surgical tracheostomy with laryngeal biopsies under general anaesthetic was carried out. Histological examination (Figs 1 and 2) demonstrated hyperplastic, hyperkeratotic squamous mucosa with reactive atypia and an underlying dense polyclonal plasmocytic inflammatory infiltrate. No granulomata, prominent eosinophils, or stigmata of vasculitis were seen. Connective tissue disease screening demonstrated normal antinuclear antibody levels with positive perinuclear anti-neutrophil cytoplasmic antibodies. Anti-proteinase 3 levels returned as 3.7 IU/ml (range 0–1.9), while antimyeloperoxidase levels were normal. Rheumatology was consulted, and a provisional diagnosis of PCM was made. Serial laryngoscopy demonstrated resolution of the oedema, with the supraglottis regaining a normal appearance despite gradual tapering of the intravenous dexamethasone. A tracheostomy capping trial was successful. The patient was decannulated uneventfully and discharged on oral prednisolone. Three months later, he was maintained on 5 mg prednisolone—attempts to taper any further caused symptoms recurrence. Outpatient referral was made to Rheumatology for medical management with steroid-sparing therapy. Unfortunately, the patient was lost to follow-up due to failure to attend for outpatient review.
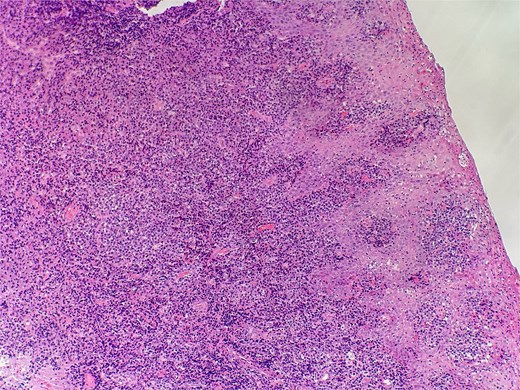
Slide from epiglottis specimen at index presentation showing squamous mucosa and underlying polyclonal plasmocytic infiltrate.
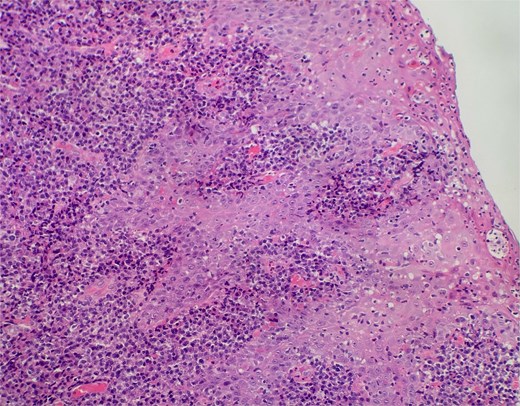
Slide from index presentation under higher magnification again demonstrating plasmocytic infiltration.
Three years later, the patient presented via ED with stridor and worsening shortness of breath. In the interim, he had tapered off steroids successfully but would frequently experience mild, episodic stridor requiring short courses of oral steroids. Worsening of stridor on the day led him to present via the ED.
On examination, the patient was in severe respiratory distress with harsh stridor and tripod stance. Flexible nasoendoscopy yielded images shown in Figs 3 and 4. Figure 3 demonstrates a retroflexed epiglottis which did not extend during respiration. Figure 4 demonstrates minimal cobblestoning of the mucosa and severe stenosis of the glottis. A CT thorax incidentally performed 2 weeks previously to monitor a lung nodule did not demonstrate any distal tracheal stenosis but did not capture the supraglottis. Further imaging was not undertaken in the acute phase.
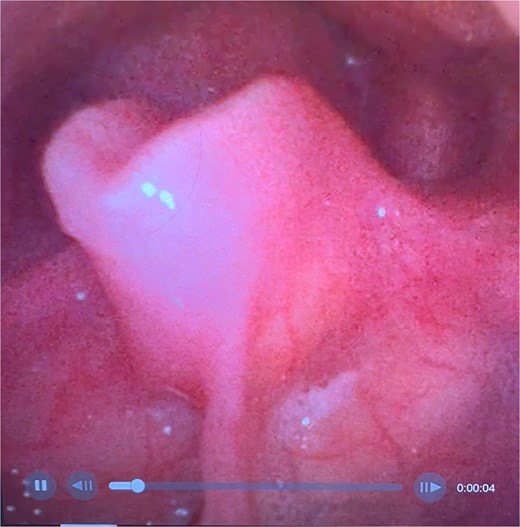
Flexible endoscopic view of a retroflexed epiglottis with a fixed, stenotic appearance of the supraglottic airway.
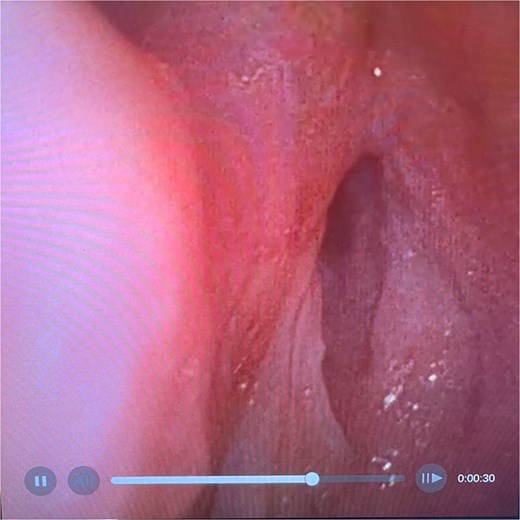
Flexible endoscopic view of best image achieved of glottic airway demonstrating severe stenosis and slit-like airway with minimal mucosal oedema.
The overall impression was of supraglottic/glottic stenosis with a tenuous airway in an otherwise healthy man. A tracheostomy was inserted under local anaesthesia through dense fibrotic tissue.
Subsequent immunological workup yielded similar results to the initial presentation and was negative for myeloma, IgG4-related systemic disease (IG4SD), or any underlying cause of plasmocytic infiltration. Referral for rheumatology review was again made. Given the isolated disease and the risks of immunosuppression, the consensus was for clinical surveillance with steroid-sparing therapy held in reserve. At follow-up at 9 months, the patient remains well but remains tracheostomy dependent. There has been no change in the stenotic appearance of the larynx.
Discussion
Permanent tracheostomy likely could have been avoided with a steroid-sparing regime; tacrolimus, methotrexate, mycophenolate mofetil, and adalimumab have all been used in this setting. Pepper et al.’s case of a lingual squamous cell carcinoma arising from PCM illustrates one argument against immunosuppression [13]. While this does not make the case for PCM representing a premalignant entity, chronic inflammation does predispose to cutaneous malignancy in the form of Marjolin ulcers [14]. Conversely, it is known that all cutaneous malignancies occur more frequently in immunosuppressed populations. In the absence of more data, symptom burden due to PCM is likely the best indicator for immunosuppression given the risks.
Supraglottic stenosis has been described secondary to supraglottoplasty in children, blunt neck trauma, intubation injury, radiotherapy, and autoimmune conditions. Siau et al. presented an interesting approach to surgical airway management in a patient with IG4SD [15]. Following recurrent stenosis after balloon dilation, Siau et al. undertook multimodal surgical management followed by medical management of rituximab and prednisolone. Surgical management for PCM is infrequently described but overrepresented with laryngeal involvement. Similarly, 20 cases of laryngeal IG4SD are reported, 13 of which needed surgical management in some form. Stepwise surgical management based on symptomatology and involved mucosa is currently the standard of care.
Conclusion
PCM is a rare diagnosis within the upper aerodigestive tract. Clinical suspicion and histological confirmation are required for diagnosis. Management is guided by the degree and location of the involved mucosa. Aggressive medical management is warranted with progression to surgical management following the identification of progressive or airway-threatening disease.
Author contributions
Concept and design: L.S. and G.P.S. Acquisition, analysis, or interpretation of data: G.P.S., K.G., and R.L. Drafting of the manuscript: G.P.S. Critical revision of the manuscript for important intellectual content: G.P.S., K.G., R.L., and L.S.
Conflict of interest statement
The authors declare that they have no conflicting/competing interests.
Funding
No funding was sought or received for this study.
Ethical approval
Approval was not sought. Patient consent was sough and is submitted separately.
Data availability
Available at request from relevant institutions.



