-
PDF
- Split View
-
Views
-
Cite
Cite
Nopparuj Sangnoppatham, Pisit Triamchaipisut, Jitpanu Wongyongsil, Phanop Limlunjakorn, Panya Thaweepworadej, Challenging diagnosis of clear cell hidradenocarcinoma of the female breast: a case report and the role of pre-operative ultrasonography in differentiating the origin of the tumor, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf491, https://doi.org/10.1093/jscr/rjaf491
Close - Share Icon Share
Abstract
Clear cell hidradenocarcinoma is a rare neoplasm of the breast that can mimic tumors originating from superficial breast tissue. Early recognition of the tumor’s origin is essential for guiding appropriate evaluation and treatment. We report a case involving a 75-year-old patient with hidradenocarcinoma of the breast and highlight the utility of preoperative ultrasonography in differentiating the tumor’s origin. Due to the limited data available on the nature of this disease, a modified radical mastectomy was performed following a wide excisional biopsy.
Introduction
Hidradenocarcinoma is a rare malignant adnexal tumor that arises from the sweat glands and accounts for < 0.001% of all tumors [1]. Although most patients with hidradenocarcinoma present with skin lesions on the face and trunk, involvement of the breast area is rare. This poses a significant diagnostic challenge as it is difficult to differentiate it from those originating from superficial breast tissue. Hence, we present our experience of a patient diagnosed with clear cell hidradenocarcinoma of the breast and discuss the utility of preoperative ultrasonography in determining origin of tumor.
Case presentation
A 75-year-old female patient presented with left nipple pain and nipple discharge for one year. She denied any family history of breast or ovarian cancer, as well as any use of tobacco or hormonal drugs. Physical examination revealed a 1.5 × 1.5 cm firm, tender, and well-circumscribed mass at the left nipple, without skin or nipple retraction. The ipsilateral axillary lymph node was not palpable. While mammography was unremarkable, ultrasonography revealed a 1.7 × 1.5 cm oval, inhomogeneous hypoechoic mass with a predominantly vascularized rim (Fig. 1). An inflammatory mass, classified as BI-RADS 3, was considered.
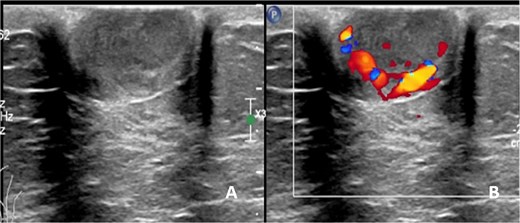
Ultrasonographic finding of left nipple mass, (A) before applying color Doppler mode and (B) after applying color Doppler mode.
After discussing treatment options with the patient, a wide excisional biopsy of the left nipple was performed. Histopathological examination revealed a margin-free, ill-defined dermal mass and no breast tissue was identified. The tumor consisted of both solid and cystic components, with an eosinophilic hyalinizing stroma. The solid areas were composed of clear cells and eosinophilic cells with vesicular nuclei and prominent nucleoli (Fig. 2). Additional immunohistochemical (IHC) staining was positive for epithelial membrane antigen (EMA), CK7, cytokeratin 19 (CK19), P16, GATA-3 (focal), smooth muscle actin (SMA) (focal), and human epidermal growth factor receptor 2 (HER2). It was negative for cytokeratin 20 (CK20), mammaglobin, Wilms' tumor 1 protein (WT1), paired-box gene 8 (PAX8), S-100, Sry-type HMG box gene 8 (SOX-8), calponin, desmin, carcinoembryonic antigen (polyclonal) (CEAp), P53, P40, P63, thyroid transcription factor-1 (TTF-1), uroplakin III, companion diagnostics (CDX), special AT-rich sequence-binding protein 2 (SATB2), as well as estrogen receptor (ER) and progesterone receptor (PR). The final pathological diagnosis confirmed that the tumor was consistent with a malignant eccrine gland tumor, favoring clear cell hidradenocarcinoma.
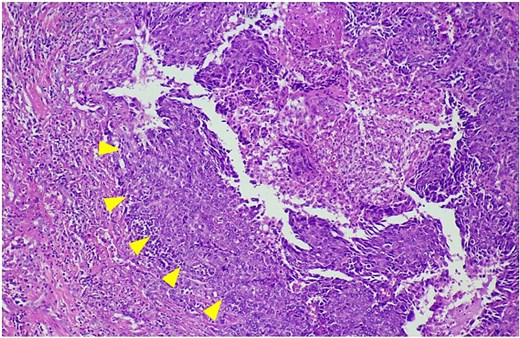
The histological features of hematoxylin and eosin staining shows clear cells and eosinophilic cells with vesicular nuclei and prominent nucleoli (arrowheads).
A computed tomography (CT) scan of the chest and upper abdomen revealed only an ipsilateral sub-centimeter lymph node, with no evidence of distant metastasis. After multidisciplinary discussion, a left modified radical mastectomy (MRM) was performed due to the uncertain nature of the disease about local recurrence and the risk of lymph node metastasis. The surgical specimen showed only fibrosis without residual malignant tumor. Additionally, there was no evidence of axillary lymph node metastasis. The postoperative course was uneventful and the patient was discharged on postoperative day 10. No adjuvant treatment was administered. As of now, 1 year later, the patient has shown no evidence of local or distant recurrence.
Discussion
Hidradenocarcinoma involving breast is an extremely rare disease entity. To date, only eight articles have documented cases of hidradenocarcinoma with breast involvement [2–9]. The clinical features and outcomes of each case are summarized in Table 1. Preoperative differentiation of this tumor from mass originating in the superficial breast parenchyma, particularly the anterior terminal duct lobular unit (TDLU), poses a diagnostic challenge. Because a definitive diagnosis of malignant adnexal tumors cannot always be made based solely on core needle biopsy [8], wide local excision may be preferred for both diagnosis and curative treatment. Therefore, preoperative prediction of the tumor’s origin is beneficial.
Main clinical features of hidradenocarcinoma of breast as reported in literature
| References . | Sex/Age . | Clinical presentations . | Duration . | Location . | Size . | Treatment . | Adjuvant treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1. Kazakov et al. [2] | M/61 | Solitary nodule | Unknown | Nipple | 2 cm | Excision | Unknown | NED at 4 years |
| 2. Giorgini et al. [3] | M/82 | Asymptomatic mass | 3 months | Left areola | 3 cm | Radical mastectomy | No | NED at 1 year |
| 3. Mezzabotta et al. [4] | F/77 | Inflammatory mass with ipsilateral lymph nodes metastasis | 3 months | Right breast | 5 cm | Right mastectomy with lymphadenectomy | Tamoxifen | NED at 38 months |
| 4. Chambers et al. [5] | F/58 | Palpable nodule | Unknown | Right areola | 1.5 cm | Lumpectomy | Unknown | Unknown |
| 5. López Rojo et al. [6] | F/76 | Palpable nodule | Unknown | Right areola | 2 cm | Simple mastectomy with SLNB | No | NED at 3 years |
| 6. An et al. [7] | F/47 | Rapidly growing mass from pre-existing nodule | 10 years | Left areola | 4 cm | Excision | RT | NED at 1 year |
| 7. Alves et al. [8] | F/92 | Palpable mass | 2 years | Left breast | 7 cm | Wide excision | No | NED at 20 months |
| 8. Kang et al. [9] | M/41 | Palpable nodule | 2 months | Right areola | 1 cm | Wide excision with SLNB | No | NED at 6 months |
| References . | Sex/Age . | Clinical presentations . | Duration . | Location . | Size . | Treatment . | Adjuvant treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1. Kazakov et al. [2] | M/61 | Solitary nodule | Unknown | Nipple | 2 cm | Excision | Unknown | NED at 4 years |
| 2. Giorgini et al. [3] | M/82 | Asymptomatic mass | 3 months | Left areola | 3 cm | Radical mastectomy | No | NED at 1 year |
| 3. Mezzabotta et al. [4] | F/77 | Inflammatory mass with ipsilateral lymph nodes metastasis | 3 months | Right breast | 5 cm | Right mastectomy with lymphadenectomy | Tamoxifen | NED at 38 months |
| 4. Chambers et al. [5] | F/58 | Palpable nodule | Unknown | Right areola | 1.5 cm | Lumpectomy | Unknown | Unknown |
| 5. López Rojo et al. [6] | F/76 | Palpable nodule | Unknown | Right areola | 2 cm | Simple mastectomy with SLNB | No | NED at 3 years |
| 6. An et al. [7] | F/47 | Rapidly growing mass from pre-existing nodule | 10 years | Left areola | 4 cm | Excision | RT | NED at 1 year |
| 7. Alves et al. [8] | F/92 | Palpable mass | 2 years | Left breast | 7 cm | Wide excision | No | NED at 20 months |
| 8. Kang et al. [9] | M/41 | Palpable nodule | 2 months | Right areola | 1 cm | Wide excision with SLNB | No | NED at 6 months |
F: female, M: male, NED: no evidence of disease, RT: radiation therapy, SLNB: sentinel lymph node biopsy.
Main clinical features of hidradenocarcinoma of breast as reported in literature
| References . | Sex/Age . | Clinical presentations . | Duration . | Location . | Size . | Treatment . | Adjuvant treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1. Kazakov et al. [2] | M/61 | Solitary nodule | Unknown | Nipple | 2 cm | Excision | Unknown | NED at 4 years |
| 2. Giorgini et al. [3] | M/82 | Asymptomatic mass | 3 months | Left areola | 3 cm | Radical mastectomy | No | NED at 1 year |
| 3. Mezzabotta et al. [4] | F/77 | Inflammatory mass with ipsilateral lymph nodes metastasis | 3 months | Right breast | 5 cm | Right mastectomy with lymphadenectomy | Tamoxifen | NED at 38 months |
| 4. Chambers et al. [5] | F/58 | Palpable nodule | Unknown | Right areola | 1.5 cm | Lumpectomy | Unknown | Unknown |
| 5. López Rojo et al. [6] | F/76 | Palpable nodule | Unknown | Right areola | 2 cm | Simple mastectomy with SLNB | No | NED at 3 years |
| 6. An et al. [7] | F/47 | Rapidly growing mass from pre-existing nodule | 10 years | Left areola | 4 cm | Excision | RT | NED at 1 year |
| 7. Alves et al. [8] | F/92 | Palpable mass | 2 years | Left breast | 7 cm | Wide excision | No | NED at 20 months |
| 8. Kang et al. [9] | M/41 | Palpable nodule | 2 months | Right areola | 1 cm | Wide excision with SLNB | No | NED at 6 months |
| References . | Sex/Age . | Clinical presentations . | Duration . | Location . | Size . | Treatment . | Adjuvant treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1. Kazakov et al. [2] | M/61 | Solitary nodule | Unknown | Nipple | 2 cm | Excision | Unknown | NED at 4 years |
| 2. Giorgini et al. [3] | M/82 | Asymptomatic mass | 3 months | Left areola | 3 cm | Radical mastectomy | No | NED at 1 year |
| 3. Mezzabotta et al. [4] | F/77 | Inflammatory mass with ipsilateral lymph nodes metastasis | 3 months | Right breast | 5 cm | Right mastectomy with lymphadenectomy | Tamoxifen | NED at 38 months |
| 4. Chambers et al. [5] | F/58 | Palpable nodule | Unknown | Right areola | 1.5 cm | Lumpectomy | Unknown | Unknown |
| 5. López Rojo et al. [6] | F/76 | Palpable nodule | Unknown | Right areola | 2 cm | Simple mastectomy with SLNB | No | NED at 3 years |
| 6. An et al. [7] | F/47 | Rapidly growing mass from pre-existing nodule | 10 years | Left areola | 4 cm | Excision | RT | NED at 1 year |
| 7. Alves et al. [8] | F/92 | Palpable mass | 2 years | Left breast | 7 cm | Wide excision | No | NED at 20 months |
| 8. Kang et al. [9] | M/41 | Palpable nodule | 2 months | Right areola | 1 cm | Wide excision with SLNB | No | NED at 6 months |
F: female, M: male, NED: no evidence of disease, RT: radiation therapy, SLNB: sentinel lymph node biopsy.
As the most common location of breast hidradenocarcinoma has been reported to be the nipple and subareolar region [2–9], mammography is often less sensitive in detecting these lesions, likely due to the increased density of the subareolar area [10]. In contrast, ultrasonography may be particularly useful in such cases. Due to its excellent spatial resolution, ultrasonography is the optimal modality for localizing superficial breast masses. Since superficial breast cancers arising from the anterior TDLU rarely occur entirely within the dermal layer, Giess et al. [10] have described the use of the dermal line identified on ultrasound to help determine the tumor’s origin. When a lesion is partially within the dermis, a ‘claw’ of dermal tissue wrapping around the margin of the lesion suggests a dermal origin (Fig. 3). This observation is based on the fact that intradermal tumors push the dermis downward, forming an acute angle with the dermal line, known as the ‘claw sign.’ In contrast, hypodermal lesions push upward against the dermis, forming an obtuse angle.
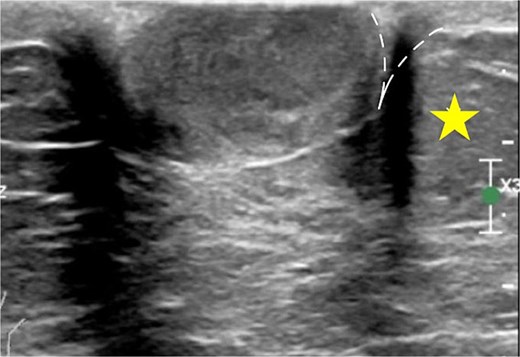
The claw sign. The ultrasonographic image shows a ‘claw’ of dermal tissue (dashed line) wrapping around the margin of the tumor and forming an acute angle. The star indicates the hypodermal layer.
Due to the conflicting results of radiotherapy (RT) and the absence of standard chemotherapy regimens, surgery remains the mainstay of treatment [1, 11]. Given the high incidence of distant metastases, clinically involved lymph node regions should be subjected to dissection [1]. In our patient, we opted for MRM due to suspicious ipsilateral axillary lymph node metastasis identified on CT. However, the final pathological report showed no evidence of metastatic axillary lymph nodes.
Our case highlights the importance of utilizing pre-operative ultrasonography to predict the origin of superficial breast masses and emphasizes the value of a multimodal approach. To our knowledge, this is the first report to describe the use of preoperative ultrasonography in breast hidradenocarcinoma. Careful and prompt investigation, thorough differential diagnosis, and well-planned surgical intervention can contribute to favorable patient outcomes.
Acknowledgements
Thank you to Dr. Suchada Hoonpongsimanont for her expert ultrasonographic review.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
Open access funding was provided by Bangkok Metropolitan Administration (BMA) General hospital.
Informed consent
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Ethics
Ethical approval is exempted for this type of publication in our institution.



