-
PDF
- Split View
-
Views
-
Cite
Cite
Mandeep Guragai, Swechha Bhatt, Satish Vaidya, Robin Man Karmacharya, Michael J Malinowski, Single-needle percutaneous foamed sclerotherapy using ultrasound and fluoroscopy guidance using “contrast washout technique” for the treatment of a slow-flow chest wall vascular malformation in a pediatric patient, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf487, https://doi.org/10.1093/jscr/rjaf487
Close - Share Icon Share
Abstract
Venous malformations (VMs) are the most common type of vascular malformation. In resource-limited settings, treating these with minimally invasive and cost-effective approaches can achieve optimal outcomes. We present the case of a 7-year-old child with a large, slow-flow VM on the right anterior chest wall, successfully treated with ultrasound- and fluoroscopy-guided foam sclerotherapy using polidocanol via the ``contrast washout'' technique. The procedure resulted in significant lesion resolution with no post-procedural pain. In addition to its effectiveness, this approach also spares patients from extensive surgical interventions, making it a valuable treatment option for pediatric VMs.
Introduction
Vascular malformations are rare disorders that can present as isolated solitary lesions, multifocal anomalies, or as part of various syndromic conditions [1]. Among these, venous malformations (VMs) are the most common congenital vascular malformations, often manifesting as soft, subcutaneous masses with a bluish-purple discoloration. These malformations may occur sporadically or have a hereditary component [2]. Despite their prevalence, the exact etiology of VMs remains incompletely understood [3]. The diagnosis of vascular malformations is frequently delayed or missed [4], particularly in resource-limited settings such as Nepal. Here, we report a case of a 4-year-old child with a right anterior chest wall VM, successfully treated with a single session of ultrasound- and fluoroscopy-guided foamed sclerotherapy using a contrast washout technique with no residual symptomatology.
Case presentation
A 7-year-old child presented with a large bluish-purple lesion on the right anterior chest wall extending to the axilla. The lesion had been present since birth and was noted by the mother to enlarge when the child cried. It was intermittently painful. The child had previously undergone evaluations at multiple healthcare centers, and a magnetic resonance angiography had confirmed the presence of a VM. No additional lesions were identified during clinical examination (Fig. 1 and Supplementary Video 1). The child’s developmental history was unremarkable, and there was no familial history of similar lesions.
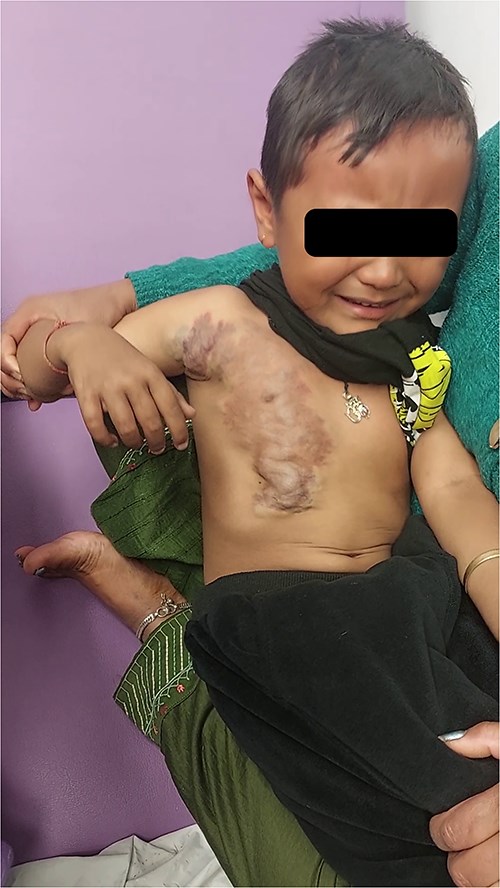
A large venous malformation on the right anterior chest wall, visibly enlarging when the child cries.
Color and spectral Doppler ultrasonography revealed a multicystic, low-flow vascular lesion (Fig. 2). Based on these findings, a diagnosis of sporadic, unifocal, slow-flow VM was established. Pre-procedure coagulation screening, including prothrombin time, and baseline renal function tests were conducted.

(A and B) Ultrasound images demonstrating cystic vascular spaces with non-pulsatile venous flow.
A single-needle direct-stick phlebography was performed using a 25-gauge butterfly cannula under ultrasound guidance. Blood aspiration confirmed the lesion's venous nature. Iopromide (Ultravist™) was administered and subsequent fluoroscopic imaging by Phillips Allura Xper FD System demonstrated slow contrast washout, further confirming the low-flow nature of the malformation (Fig. 3). Minimal venous drainage was observed, consistent with Puig Class I categorization [5].
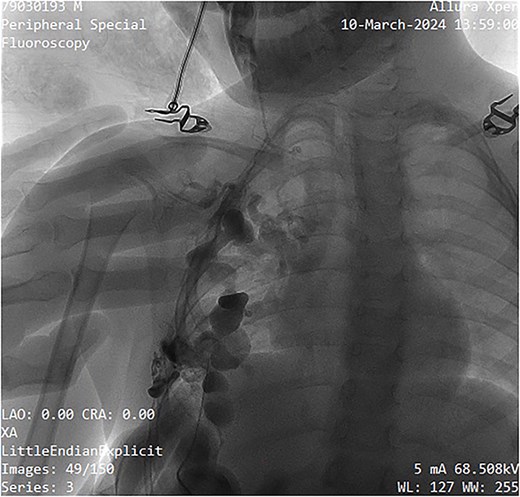
Fluoroscopy image showing contrast administration into the malformation, confirming its slow-flow nature, and drainage pattern.
The sclerosing agent used was 3% polidocanol foam, prepared via Tessari’s technique. The foam formulation comprised 3 ml of polidocanol, 3 ml of normal saline, and 3 ml of air, totaling 9 ml. The non-radiopaque polidocanol was administered through the same needle. The contrast was subsequently ``washed out'' by the sclerosing agent, ensuring comprehensive treatment of all cystic spaces (Fig. 4). The procedure was done as a day case under local anesthesia with a support staff calming the patient. Pre- and post-procedure digital subtraction angiography images were obtained for mapping and completion. Post-instillation ultrasonography was performed to confirm adequate sclerotherapy distribution and to confirm extravasation did not occur. Compression taping was applied post-procedure, and the patient was discharged within a few hours on oral paracetamol as required.
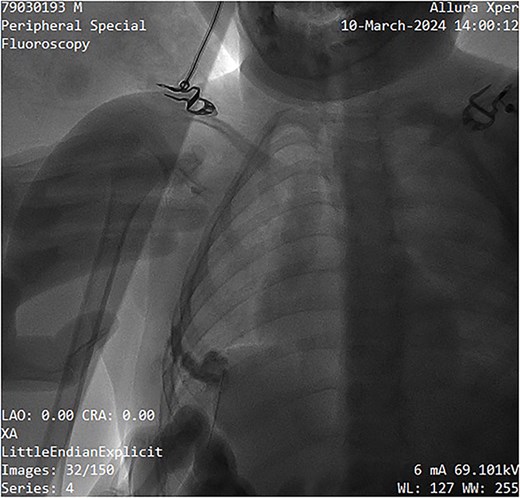
Image showing sclerotherapy administration through the same needle used for contrast injection, effectively ``washing out'' the contrast while sclerosing the venous malformation.
At the 2-month follow-up, significant resolution of the swelling was observed, with only minor residual fullness noted in the inframammary region of the right chest wall (Fig. 5). Ultrasonography revealed sclerosed vascular channels without flow (Fig. 6). The patient did not experience post-procedural pain. The parents were satisfied with the outcome with no residual symptomatology reported in their child since the time of the single session treatment. They ultimately opted for possible additional sclerotherapy sessions at a later date to address any residual malformation as needed.
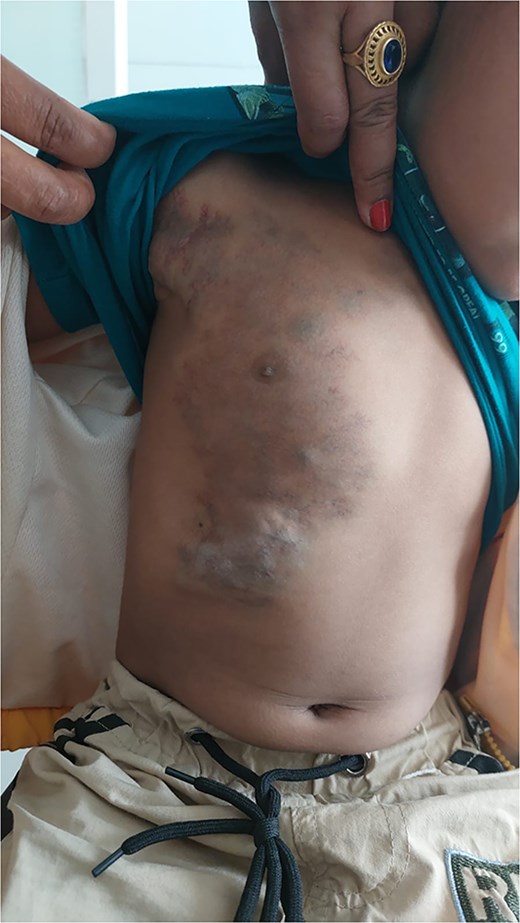
Patient at 2-month follow-up demonstrating significant resolution of the venous malformation.
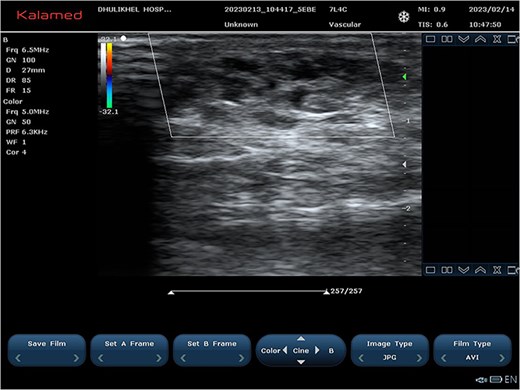
Ultrasound images at 2-month follow-up showing sclerosed vascular channels without flow.
Discussion
Sporadic slow-flow VMs can be managed using various treatment modalities, including sclerotherapy, surgical excision, radiofrequency ablation, or a combination of these approaches [6]. Among these, sclerotherapy is widely recognized as a safe and effective option for pediatric patients [7], particularly for Puig type I and II lesions [5]. Several sclerosing agents, such as bleomycin, sodium tetradecyl sulfate, and polidocanol, have demonstrated favorable outcomes in the treatment of VMs [8]. In our case, we utilized 3% polidocanol as the sclerosing agent due to its availability at our center and its well-documented safety and efficacy profile, with a lower risk of complications compared to other agents [9]. Its safety and effectiveness have been further established in the treatment of oral vascular malformations, including those affecting the tongue [10].
Although sclerotherapy is generally safe, serious complications, including skin ulceration, post-procedural pain, dysrhythmias, and strokes, have been documented in the literature [11]. However, such adverse events are rare in slow-flow malformations. To minimize the risk of complications, we limit the administered volume of polidocanol to maximal 10 ml per session [12], ensure the exclusion of high-flow vascular malformations prior to treatment, and confirm minimal venous drainage into deep systemic veins through phlebography prior to procedure.
Ultrasound plays a crucial role in the evaluation and management of vascular malformations. VMs typically exhibit a heterogeneous uni- or multilocular structure with cystic areas that may or may not be compressible, in addition to the presence of phleboliths, which are considered pathognomonic [13]. Pre-procedural ultrasound assessment provides valuable information regarding lesion accessibility for percutaneous needle placement, selection of appropriate treatment modalities (sclerotherapy vs. alternative interventions) [13], and evaluation of surrounding venous structures [14]. Furthermore, ultrasound can serve as an objective tool for monitoring treatment response in pediatric patients undergoing sclerotherapy [15]. However, ultrasound is highly subjective and operator dependent, therefore, reliance on a single imaging modality for diagnosis may be insufficient. A multimodal diagnostic approach incorporating clinical examination, ultrasound, phlebography, and magnetic resonance imaging enhances diagnostic accuracy and optimizes treatment planning [16].
The combination of ultrasound and fluoroscopy guidance during percutaneous sclerotherapy is a highly effective approach in the management of VMs. Bagga et al. identified four distinct phlebographic patterns of VMs: cavitary, spongy, dysplastic vein, and mixed patterns [14]. Their study, which evaluated prognostic indicators for successful sclerotherapy outcomes, found that cystic vascular lesions on ultrasound demonstrated the highest rates of favorable response [14]. Our case involved a lesion with a predominantly cavitary pattern on phlebography, and our findings align with their results.
The standard percutaneous technique for treating VMs involves a double-needle approach, wherein a second needle is employed for aspiration to prevent sclerosant extravasation and ensure optimal endothelial contact with the sclerosing agent [17]. In this case, we utilized a single-needle approach under ultrasound guidance, ensuring precise intracystic placement of the needle tip. Fluoroscopy confirmed lesion morphology and the slow-flow nature of the malformation. The contrast washout technique allowed for visualization of the sclerosant displacement and ensured complete treatment coverage. Post-procedural ultrasound assessment verified adequate distribution of the sclerosing agent, and immediate compression with elastic tape was applied to optimize therapeutic efficacy. We propose that this single-needle technique serves as a viable alternative to the conventional double-needle approach, particularly for the treatment of isolated slow-flow malformations.
Assessing treatment outcomes for vascular malformations remains a clinical challenge. Moussa et al. advocate for both objective and subjective evaluation methods, incorporating standardized questionnaires with predefined parameters for pre- and post-treatment assessment [18]. Objective outcome measures, including reduction in lesion size, increased echogenicity, and diminished cystic spaces, were successfully confirmed via post-treatment ultrasound in our case. However, subjective patient-reported satisfaction is equally important in determining therapeutic success. In our case, the mother of the pediatric patient expressed satisfaction with the treatment outcome, highlighting the significance of patient and caregiver perspectives in assessing clinical efficacy.
Conclusion
Ultrasound- and fluoroscopy-guided single-needle percutaneous foamed sclerotherapy using contrast washout technique is a safe and effective method for treating low-flow VMs in pediatric patients. This technique allows for precise lesion characterization and comprehensive treatment while minimizing procedural morbidity. Further studies are warranted to establish standardized protocols for optimal dosing and agent selection in pediatric vascular malformation management. The limited use of resources and resolution of symptoms and pain, especially in a pediatric cohort, offer high value per cost for the technique in practices that treat vascular malformations in low-income countries.
Conflict of interest statement
None declared.
Funding
None declared.



