-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos Eduardo Duque, Lelys Arévalo-Valdivieso, Cristian Reino, Alejandro Gallegos-Correa, Daniel Peñaherrera-Vásquez, Luis Fuenmayor-González, AI-assisted prostheses manufacturing for cranioplasties in low-income countries. A case report and technique description, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf482, https://doi.org/10.1093/jscr/rjaf482
Close - Share Icon Share
Abstract
Cranioplasty restores skull integrity after decompressive craniectomy, but high costs limit access in low-resource settings. This case highlights an artificial intelligence (AI)-assisted design method for a low-cost, patient-specific cranial prosthesis. A 15-year-old male with a traumatic brain injury and left intraparenchymal hematoma underwent cranioplasty complicated by infection and necrosis. A second procedure obtained positive outcomes using a custom AI-designed prosthesis generated from computed tomography imaging, 3D modeling, and polymethylmethacrylate fabrication. AI-enabled modeling ensured precise anatomical fit, reducing intraoperative adjustments and overcoming limitations of manual techniques. This approach enhances surgical outcomes and prosthesis accessibility in resource-limited settings. AI improves the design of cranial prostheses, lowering costs and production time. Its application could be cost-effective, especially in low-resource settings.
Introduction
Cranioplasty is a widely performed neurosurgical procedure for reconstructing cranial defects following decompressive craniotomy. While autologous bone remains the preferred option, it is not always available [1, 2].
Polymethylmethacrylate (PMMA) is one of the most commonly used allogenic materials for cranial reconstruction due to its reasonable biocompatibility, availability, low cost, strength, and ability to be premolded. However, achieving the correct shape and curvature through manual molding can be challenging. For this reason, industrial 3D printing, guided by data-driven methods, is often employed to produce well-fitted implants [1, 3].
Some authors suggest that data-driven approaches, including artificial intelligence (AI) techniques such as machine learning and deep learning, alongside publicly available clinical databases, will contribute to developing the next generation of patient-specific cranial implants, potentially improving clinical outcomes [2, 4].
This case report is particularly relevant as the custom prosthesis was designed with AI guidance. Unlike previous studies that relied on specialized software or parametrized continuous occupation functions, such as neural networks, this approach aims to demonstrate a clinically effective technique while reducing costs and production time, making it a feasible option for low-resource settings. This case was reported using the Surgical Case Reports (SCARE) guidelines [5].
Case report
A 15-year-old mestizo male with no significant medical history sustained a traumatic brain injury in a traffic accident in October 2023. He was transported by ambulance to a tertiary hospital in Guayaquil with a Glasgow Coma Scale score of 6 (M3V2O1), cerebrospinal fluid (CSF) leakage, epistaxis, left zygomatic abrasions, and a frontal scar exposing the fracture line. No signs of intracranial hypertension or cerebral edema were observed.
A non-contrast cranial computed tomography (CT) scan (Fig. 1) revealed fractures of the orbital roof, nasal bones, and left zygomatic bone, as well as a frontal fracture, intraparenchymal hemorrhages in the parietal and temporal lobes, and hemorrhage within the paranasal sinuses. Laboratory tests were unremarkable.
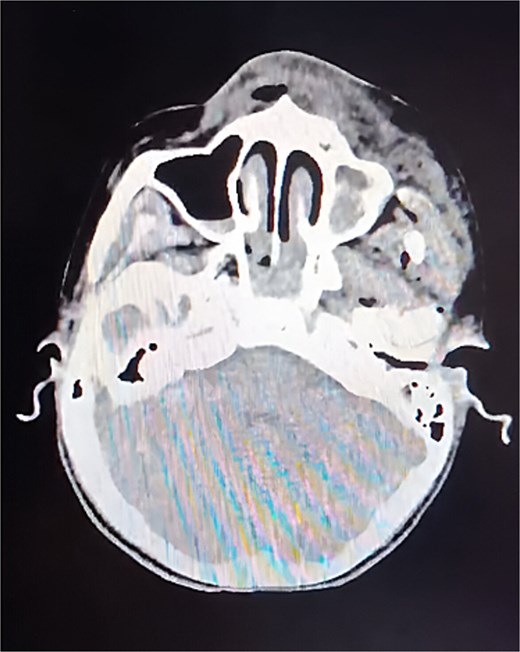
CT of the skull, axial slice. A cranial base fracture is evident, with bleeding at the level of the maxillary sinus. Small areas of bleeding are also observed in the intraparenchymal region.
Due to the diagnosis of a left intraparenchymal hematoma, a left frontoparietotemporal decompressive craniotomy and cranioplasty were performed in February 2024. Within a month, the patient developed a CSF fistula, signs of infection, skin tissue lysis, chronic inflammatory changes, and full-thickness scalp necrosis with bacterial contamination (Fig. 2). These complications were attributed to a foreign body reaction to the cranioplasty material.
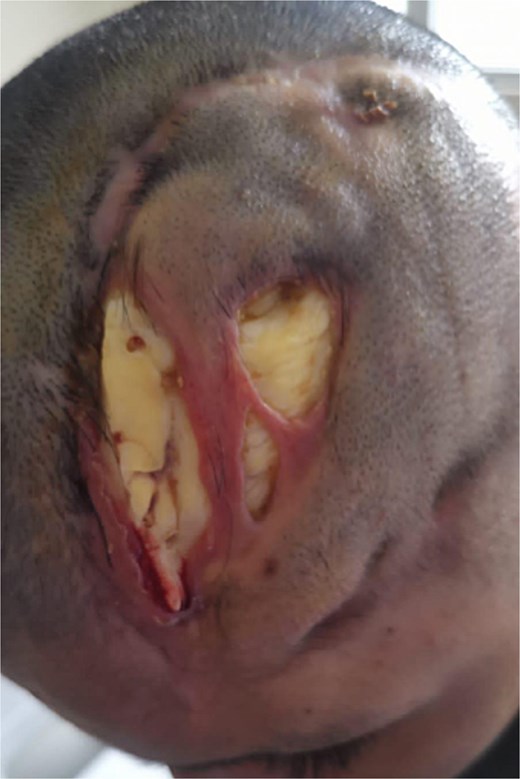
Preoperative image. Tissue lysis at the scalp level, exposing the cement prosthesis or previous surgical procedure.
The patient’s mother reported that she had discontinued follow-up at the original institution and sought a new evaluation with our team in March 2024. The patient received continuous follow-up until July 2024, when he was admitted to the tertiary-level Nueva Aurora-Luz Elena Arismendi Obstetric and Pediatric Hospital (HGONA by its initials in Spanish) (See timeline at Supplementary Appendix 1).
At the new institution, a second cranioplasty was planned, utilizing an AI-guided prosthesis design to create a fully customized implant while minimizing fabrication costs. For this purpose, a multidisciplinary approach was chosen, using computer-aided design tools, a Python programming language algorithm for the training of the AI, and medical image-specialized processing software. The entire process of the AI-guided prosthesis design is described in Supplementary Appendix 2. Different materials were used for this process, and reference costs are shown in Supplementary Appendix 3.
Once the prosthesis was created, the second reconstructive cranioplasty was performed. Dr. A-V.L. (a neurosurgeon with four years of experience) began the procedure by debriding the necrotic skin and executing an expanded pterional approach. She then removed the irregularly edged bone cement graft that was in contact with the cerebral parenchyma and took a sample of the purulent secretion beneath the dura mater. The dura mater was subsequently irrigated with two liters of Ringer’s Lactate solution, followed by suturing and sealing. Finally, the PMMA graft was placed and secured with self-tapping screws and three 14 mm titanium plates (Fig. 3).
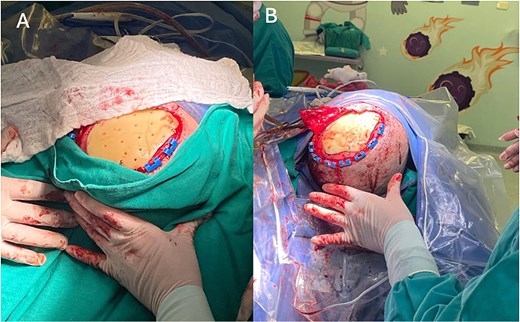
Surgical procedure. (A) Placement and fixation of the PMMA prosthesis with titanium plates and screws. (B) Layered closure and creation of a rotation fasciocutaneous flap.
Dr. R.C., a plastic surgeon with four years of experience, created a scalp rotation flap with an axial pedicle, preserving the aponeurotic plane. After elevating the flap, it was transposed to cover the PMMA graft and address the cutaneous defect. A full-thickness skin graft was harvested from the thigh to cover the donor site of the scalp flap, and both the flap and graft were sutured in layers (Fig. 4).
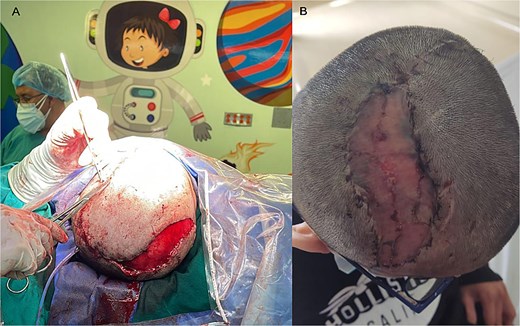
Coverage of PMMA prosthesis. (A) A large-dimensional rotational skin flap was created to cover the entire surface of the prosthesis and osteosynthesis material. Skin grafts were then performed over the residual donor area. (B) Postoperative follow-up for suture removal, 15 days later, observing complete viability of the flap and graft area.
The total length of the surgery was 4 hours, including anesthesia time, and was directly related to the complexity of the approach and the need to perform multiple complementary procedures.
A CT scan after the surgery revealed normal cerebral parenchyma; however, the patient exhibited right-hand paresis ++/++++ and a subcutaneous CSF collection with no communication to the exterior, which was resolved with acetazolamide administration. The patient had a good overall recovery, with successful bone integration and no postoperative complications, achieving full recovery of his lifestyle and regular school and recreational activities (Fig. 5).
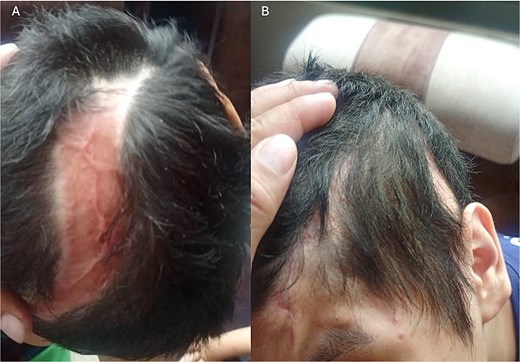
Postoperative follow-up, 4 months after surgery. (A) Flap and graft area with adequate healing; an alopecic area is visible, which will be treated one year after surgery for the placement of tissue expanders. (B) Site of prosthesis placement with an appropriate healing process.
Discussion
Defects in the bones of the cranium may directly impact the structures within the cranial vault and lead to esthetic concerns [6, 7]. Ideally, autologous bone should be used for this type of reconstruction; however, it is not always available. In such cases, alternative synthetic materials are utilized, including metals, ceramics, plastics, resorbable polymers, and other biomaterials [8]. The implant must be appropriate in shape and size for the cranial defect, biocompatible, inert, thermally non-conductive, radiotransparent, non-magnetic, rigid, easy to place, and ideally low-cost [8, 9].
The PMMA powder can be molded manually during surgery or preoperatively. In our case, the PMMA implant was created using a 3D printer mold before surgery [1, 3]. Manual molding is suitable for reconstructing minor defects or when the original bone from the craniectomy is available. However, this method may complicate obtaining an adequate contour for larger defects, potentially leading to unsatisfactory esthetic outcomes [10, 11].
Molding usually uses customized industrial 3D printers that rely on data-driven methods to achieve the correct implant shape. The process involves uploading the patient’s CT scan to a computer to create an implant model with specialized software or continuous parameterized neural networks. The data is then sent to a 3D printer to fabricate the implant [2–4].
Although the functional, esthetic, and economic outcomes reported in the literature are comparable to those in our case, the main difference lies in the technological approach. Whereas many studies utilize specialized software to model the prosthesis, our case implemented AI. This distinction is significant because AI enables continuous learning, automates model generation from large datasets, and reduces manual intervention, thereby optimizing time, anatomical accuracy, and adaptability in complex cases [12].
Most studies involving 3D-printed cranial implants guided by traditional software report surgical durations ranging from 90 to 180 minutes. In contrast, although the total surgical time in our case was 4 hours due to additional procedures performed—such as the rotational flap, dural plasty, and skin graft—the cranioplasty itself was completed in approximately 60 minutes. This represents a notably shorter duration compared to what is typically reported in the literature. The reduced time reflects the enhanced efficiency of AI-driven workflows, which automate and optimize implant design beyond the capabilities of conventional software. Consequently, this reduction in operative time contributes to decreased overall costs by minimizing operating room occupancy and associated resources [13–15].
As one of the pioneers in using AI for such prostheses, this project faced challenges; however, the knowledge generated and shared in this report is believed to further optimize time and resources.
This case report outlines an efficient technique for manufacturing cranioplasty prostheses. The customized implant developed through this methodology demonstrated a precise anatomical fit during the surgical procedure. This not only improved the patient’s cranial esthetics but also reduced operating time by eliminating the need for further intraoperative adjustments.
Strengths and limitations
The positive outcomes observed in this study resulted from excellent multidisciplinary collaboration between the neurosurgery, plastic surgery, and biomedical engineering teams responsible for creating the prosthesis. However, some complications occurred, primarily due to the limited information obtained from the institution where the patient underwent the first reconstructive cranioplasty.
Patient mother’s perspective
“I am deeply grateful to the medical team for their unwavering support during my son’s surgery. Thanks to their dedication and expertise, he has made an excellent recovery and successfully reintegrated into his studies and daily activities. His well-being is a testament to the high-quality medical care and compassionate support he received”.
Conclusion
Cranioplasty is a neurosurgical procedure designed to enhance structural and functional outcomes. The properties of the prosthesis are essential for achieving these results. This AI-assisted methodology is straightforward, safe, and cost-effective in terms of time and expense, and it has the potential to be replicated in low-resource countries.
Author contributions
D.C.E.: Conceptualization, Investigation, Methodology, Project Administration, Software, Validation, Writing—original draft preparation, and Writing—review & editing. A.-V.L.: Investigation, Data curation, Resources, Supervision, and Writing—original draft preparation. R.C.: Investigation, Data curation, Resources, Supervision, and Writing—original draft preparation. G.-C.A.: Investigation, Methodology, Project Administration, Software, validation, and Writing—original draft preparation. P.-V.D.: Investigation, Data curation, Resources, Supervision, and Writing—original draft preparation. F.-G.L: Conceptualization, Investigation, Methodology, Project Administration, Validation, Data curation, Resources Writing—original draft preparation, and Writing—review & editing.
Conflict of interest statement
None declared.
Funding
The authors received no financial support for this article’s research, authorship, and publication.
Data availability
The specifications of materials and the code for training the AI are available upon reasonable request to the authors.
Informed consent
Written informed consent was obtained from the patient’s mother for the anonymized information to be published in this article.



